Journal of Organometallic Chemistry ( IF 2.1 ) Pub Date : 2020-06-13 , DOI: 10.1016/j.jorganchem.2020.121371 Nikolaj Тurovskij , Elena Raksha , Yuliya Berestneva , Alexander Eresko
|
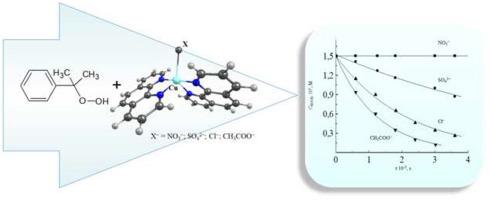
Kinetics of the cumene hydroperoxide catalytic decomposition in the presence of Cu(II) salt complexes with 1,10-phenanthroline (Phen) has been investigated in the water: ethanol (1:1) solvent mixture. Catalytic properties of Cu(CH3COO)2 - Phen complex were confirmed: after full hydroperoxide conversion its new portion had been added to the reaction mixture. The hydroperoxide decomposition rate constant observed on the second cycle was the same. The salt anion effect on the complex catalytic activity has been revealed. The rate constant of the cumene hydroperoxide catalytic decomposition increases in the following row of anions: SO42− < Cl− < CH3COO−, the hydroperoxide decomposition in the presence of Cu(II) nitrate in the same experimental conditions was not observed. The effect of the anion nature in the reaction of cumene hydroperoxide catalytic decomposition may be associated with both the structure of the Cu(II) complex with Phen, as well as the structure of the hydroperoxide - catalyst complex. In addition, the double activation effect is observed in the reaction considered. The hydroperoxide molecule is chemically activated by the corresponded Cu(ІІ) salt. The Cu(ІІ) salt reactivity by-turn is activated in the presence of 1,10-phenanthroline and can be regulated by means of variation in corresponded salt anion.
中文翻译:

阴离子对1,10-菲咯啉酸铜(II)存在下氢过氧化枯烯的分解
在水:乙醇(1:1)溶剂混合物中,研究了在Cu(II)盐与1,10-菲咯啉(Phen)配合物存在下氢过氧化枯烯催化分解的动力学。证实了Cu(CH 3 COO)2-苯配合物的催化性能:完全过氧化氢转化后,其新部分已添加至反应混合物中。在第二个循环中观察到的氢过氧化物分解速率常数是相同的。已经揭示了盐阴离子对复合催化活性的作用。的过氧化氢异丙苯催化分解增加阴离子的下面行中的速率常数:SO 4 2- <氯- <CH 3 COO -,在相同的实验条件下未观察到在硝酸铜(II)存在下氢过氧化物的分解。异丙苯氢过氧化物催化分解反应中阴离子性质的影响可能与Cu(II)与Phen的配合物的结构以及氢过氧化物-催化剂的配合物的结构有关。另外,在所考虑的反应中观察到双重活化作用。氢过氧化物分子被相应的铜盐化学活化。由转动的铜(ІІ)盐的反应性在1,10-菲咯啉的存在下活化,并且可以通过变化的方式管理在对应盐阴离子。









































 京公网安备 11010802027423号
京公网安备 11010802027423号