Food Research International ( IF 8.1 ) Pub Date : 2020-06-15 , DOI: 10.1016/j.foodres.2020.109440 Felipe Rodrigues Nogueira Silva , Amanda Dambrós Pereira , Débora Parra Baptista , Mararlene Ulberg Pereira , Bernardete Ferraz Spisso , Mirna Lúcia Gigante , Patrícia Aparecida de Campos Braga , Félix Guilhermo Reyes Reyes , Adriana Pavesi Arisseto-Bragotto
|
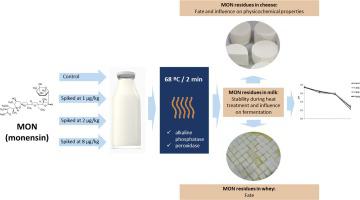
Considering the widespread use of the antibiotic monensin (MON) in the Brazilian livestock and the possibility of residues in milk, this paper aimed to study the stability and fate of this drug during the production of Brazilian Minas Frescal cheese, its effects on milk fermentation and on the physicochemical characteristics of this product. For that, samples of raw milk were fortified with MON at three different nominal concentrations (1.0, 2.0 and 8.0 μg/kg), passed through heat treatment and used to produce Minas Frescal cheese. Pasteurization efficiency was certified by alkaline phosphatase and peroxidase enzyme tests and cheese samples were evaluated for pH, moisture and total protein and fat content. MON residues were determined by LC-MS/MS in the following steps: raw milk, heat-treated milk, whey and cheese. No significant degradation of MON due to heat treatment was observed, suggesting that the drug is resistant to high temperatures. Moreover, the residue levels quantified in cheese and whey demonstrated a concentration of this antibiotic in the curd by about 5-fold, with a small amount of MON being lost during draining. There were no significant differences (p > 0.05) considering the physicochemical parameters evaluated in cheese samples. Fermentation was also not affected by the presence of the drug. The results showed that residues of MON in milk are stable during cheese production and may be concentrated in the final product, as well as indicate the need to establish a MON safe residue level for this food commodity.
中文翻译:

Minas Frescal奶酪生产中的莫能菌素残留:稳定性,对奶酪的发酵,命运和理化特性的影响
考虑到抗生素莫能菌素(MON)在巴西牲畜中的广泛使用以及牛奶中残留的可能性,本文旨在研究该药物在巴西Minas Frescal奶酪生产过程中的稳定性和命运,其对牛奶发酵和关于该产品的理化特性。为此,将生牛奶样品用三种标称浓度(1.0、2.0和8.0μg/ kg)的MON强化,经过热处理并用于生产Minas Frescal奶酪。碱性磷酸酶和过氧化物酶测试证明了巴氏杀菌的效率,并评估了奶酪样品的pH,水分以及总蛋白质和脂肪含量。MON残留物通过以下步骤的LC-MS / MS测定:生乳,热处理乳,乳清和奶酪。没有观察到由于热处理引起的MON的显着降解,表明该药物对高温具有抵抗力。此外,在奶酪和乳清中定量的残留水平表明,这种抗生素在凝乳中的浓度约为5倍,排水过程中损失了少量的MON。考虑到奶酪样品中评估的理化参数,没有显着差异(p> 0.05)。发酵也不受药物存在的影响。结果表明,牛奶中的MON残留在奶酪生产过程中是稳定的,并且可能集中在最终产品中,也表明需要为此食品确定MON安全残留水平。奶酪和乳清中定量的残留水平表明,这种抗生素在凝乳中的浓度约为5倍,排水过程中会损失少量的MON。考虑到奶酪样品中评估的理化参数,没有显着差异(p> 0.05)。发酵也不受药物存在的影响。结果表明,牛奶中的MON残留在奶酪生产过程中是稳定的,并且可能集中在最终产品中,也表明需要为此食品确定MON安全残留水平。奶酪和乳清中定量的残留水平表明,这种抗生素在凝乳中的浓度约为5倍,排水过程中损失了少量的MON。考虑到奶酪样品中评估的理化参数,没有显着差异(p> 0.05)。发酵也不受药物存在的影响。结果表明,牛奶中的MON残留在奶酪生产过程中是稳定的,并且可能集中在最终产品中,也表明需要为此食品确定MON安全残留水平。05)考虑奶酪样品中评估的理化参数。发酵也不受药物存在的影响。结果表明,牛奶中的MON残留在奶酪生产过程中是稳定的,并且可能集中在最终产品中,也表明需要为此食品确定MON安全残留水平。05)考虑奶酪样品中评估的理化参数。发酵也不受药物存在的影响。结果表明,牛奶中的MON残留在奶酪生产过程中是稳定的,并且可能集中在最终产品中,也表明需要为此食品确定MON安全残留水平。



























 京公网安备 11010802027423号
京公网安备 11010802027423号