Cell ( IF 45.5 ) Pub Date : 2020-06-15 , DOI: 10.1016/j.cell.2020.05.020 Hongwu Qian 1 , Xuelan Wu 2 , Ximing Du 3 , Xia Yao 1 , Xin Zhao 4 , Joyce Lee 1 , Hongyuan Yang 3 , Nieng Yan 1
|
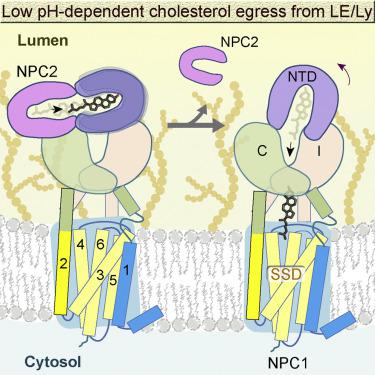
Lysosomal cholesterol egress requires two proteins, NPC1 and NPC2, whose defects are responsible for Niemann-Pick disease type C (NPC). Here, we present systematic structural characterizations that reveal the molecular basis for low-pH-dependent cholesterol delivery from NPC2 to the transmembrane (TM) domain of NPC1. At pH 8.0, similar structures of NPC1 were obtained in nanodiscs and in detergent at resolutions of 3.6 Å and 3.0 Å, respectively. A tunnel connecting the N-terminal domain (NTD) and the transmembrane sterol-sensing domain (SSD) was unveiled. At pH 5.5, the NTD exhibits two conformations, suggesting the motion for cholesterol delivery to the tunnel. A putative cholesterol molecule is found at the membrane boundary of the tunnel, and TM2 moves toward formation of a surface pocket on the SSD. Finally, the structure of the NPC1-NPC2 complex at 4.0 Å resolution was obtained at pH 5.5, elucidating the molecular basis for cholesterol handoff from NPC2 to NPC1(NTD).
中文翻译:

NPC1和NPC2的低pH依赖性溶酶体胆固醇流出的结构基础。
溶酶体胆固醇的排出需要两种蛋白质NPC1和NPC2,其缺陷是造成C型Niemann-Pick疾病的原因。在这里,我们目前的系统结构表征揭示了低pH依赖性胆固醇从NPC2传递至NPC1跨膜(TM)域的分子基础。在pH 8.0时,在纳米光盘和去污剂中分别获得3.6 N和3.0 A分辨率的NPC1类似结构。揭示了连接N末端域(NTD)和跨膜固醇感应域(SSD)的隧道。在pH 5.5时,NTD表现出两种构象,表明胆固醇向通道输送的运动。在隧道的膜边界发现了一个假定的胆固醇分子,TM2向SSD上的表面袋形成。最后,











































 京公网安备 11010802027423号
京公网安备 11010802027423号