当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mixed conductive composites for ‘Low-Temperature’ thermo-chemical CO2 splitting and syngas generation
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2020-06-12 , DOI: 10.1039/d0ta03232h Qiongqiong Jiang 1, 2, 3, 4, 5 , Yunfei Gao 1, 2, 3 , Vasudev Pralhad Haribal 1, 2, 3, 4, 5 , He Qi 3, 6, 7 , Xingbo Liu 3, 6, 7 , Hui Hong 4, 5, 8, 9, 10 , Hongguang Jin 4, 5, 8, 9, 10 , Fanxing Li 1, 2, 3
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2020-06-12 , DOI: 10.1039/d0ta03232h Qiongqiong Jiang 1, 2, 3, 4, 5 , Yunfei Gao 1, 2, 3 , Vasudev Pralhad Haribal 1, 2, 3, 4, 5 , He Qi 3, 6, 7 , Xingbo Liu 3, 6, 7 , Hui Hong 4, 5, 8, 9, 10 , Hongguang Jin 4, 5, 8, 9, 10 , Fanxing Li 1, 2, 3
Affiliation
|
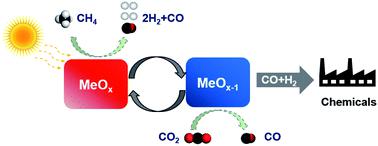
An effective strategy to design platinum group metal (PGM) free redox catalysts for “low temperature” CO2 splitting followed with methane partial oxidation was proposed and validated. Composites of mixed ionic-electronic conductive (MIEC) oxides were found to be highly effective at relatively low temperatures (600–750 °C). Specifically, perovskite structured LaNi0.35Fe0.65O3 and rock salt structured Ce0.85Gd0.1Cu0.05O2−δ, as two compatible yet structurally distinct MIEC oxides, were integrated into composite redox catalyst particles. Resulting from the synergistic effect of the two MIEC phases, 90% CO2 to CO conversion was demonstrated at 750 °C. Up to 90% methane conversion with 96% CO selectivity was also achieved in the methane POx step. The redox catalysts were characterized in detail to illustrate the underlying mechanisms for the synergistic effects. Electrical conductivity relaxation (ECR) measurements indicated significantly lowered activation energy for lattice oxygen (O2−) migration (0.43 eV). The enhanced oxygen migration in turn led to reversible exsolution of active transition metal nanoparticles (Ni–Fe alloy) from the mixed oxide, serving as active sites for methane activation while further enhancing lattice oxygen exchange, as confirmed by in situ X-ray diffraction and transmission electron microscopy. As a result, the composite redox catalysts demonstrate superior redox activity, coke resistance, and long term redox stability, making them potentially suitable for CO2 utilization and methane partial oxidation under a hybrid redox process scheme.
中文翻译:

用于“低温”热化学分解二氧化碳和合成气的混合导电复合材料
提出并验证了一种有效的策略来设计无铂族金属(PGM)的氧化还原催化剂,以实现“低温” CO 2裂解和甲烷部分氧化。发现混合离子-电子导电(MIEC)氧化物的复合物在相对较低的温度(600-750°C)下非常有效。具体而言,将钙钛矿结构的LaNi 0.35 Fe 0.65 O 3和岩盐结构的Ce 0.85 Gd 0.1 Cu 0.05 O 2- δ作为两种相容但结构上不同的MIEC氧化物,整合到复合氧化还原催化剂颗粒中。由于两个MIEC相的协同效应,所以90%CO 2在750°C下证明了向CO的转化。在甲烷POx步骤中,还可实现高达90%的甲烷转化率和96%的CO选择性。详细描述了氧化还原催化剂,以说明协同作用的潜在机理。电导率弛豫(ECR)测量表明,晶格氧(O 2−)迁移的活化能大大降低(0.43 eV)。增强的氧迁移反过来导致混合氧化物中的活性过渡金属纳米颗粒(Ni-Fe合金)可逆地溶出,这是甲烷活化的活性位点,同时进一步增强了晶格氧交换,这已通过原位证实X射线衍射和透射电子显微镜。结果,复合氧化还原催化剂表现出优异的氧化还原活性,耐焦炭性和长期氧化还原稳定性,使其潜在地适用于混合氧化还原工艺方案下的CO 2利用和甲烷部分氧化。
更新日期:2020-07-07
中文翻译:

用于“低温”热化学分解二氧化碳和合成气的混合导电复合材料
提出并验证了一种有效的策略来设计无铂族金属(PGM)的氧化还原催化剂,以实现“低温” CO 2裂解和甲烷部分氧化。发现混合离子-电子导电(MIEC)氧化物的复合物在相对较低的温度(600-750°C)下非常有效。具体而言,将钙钛矿结构的LaNi 0.35 Fe 0.65 O 3和岩盐结构的Ce 0.85 Gd 0.1 Cu 0.05 O 2- δ作为两种相容但结构上不同的MIEC氧化物,整合到复合氧化还原催化剂颗粒中。由于两个MIEC相的协同效应,所以90%CO 2在750°C下证明了向CO的转化。在甲烷POx步骤中,还可实现高达90%的甲烷转化率和96%的CO选择性。详细描述了氧化还原催化剂,以说明协同作用的潜在机理。电导率弛豫(ECR)测量表明,晶格氧(O 2−)迁移的活化能大大降低(0.43 eV)。增强的氧迁移反过来导致混合氧化物中的活性过渡金属纳米颗粒(Ni-Fe合金)可逆地溶出,这是甲烷活化的活性位点,同时进一步增强了晶格氧交换,这已通过原位证实X射线衍射和透射电子显微镜。结果,复合氧化还原催化剂表现出优异的氧化还原活性,耐焦炭性和长期氧化还原稳定性,使其潜在地适用于混合氧化还原工艺方案下的CO 2利用和甲烷部分氧化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号