当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Activating the lattice oxygen in (Bi0.5Co0.5)2O3 by vacancy modulation for efficient electrochemical water oxidation
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2020-06-12 , DOI: 10.1039/d0ta03411h Huan Liu 1, 2, 3, 4, 5 , Xiaoning Li 3, 4, 5, 6, 7 , Cailing Peng 1, 2, 3, 4, 5 , Liuyang Zhu 1, 2, 3, 4, 5 , Yuanxi Zhang 1, 2, 3, 4, 5 , Huiru Cheng 3, 4, 5, 8 , Jiameng Cui 1, 2, 3, 4, 5 , Qingmei Wu 1, 2, 3, 4, 5 , Yingying Zhang 1, 2, 3, 4, 5 , Zezhi Chen 1, 2, 3, 4, 5 , Wei Zou 1, 2, 3, 4, 5 , Wen Gu 1, 2, 3, 4, 5 , Haoliang Huang 3, 4, 5, 6, 7 , Jianlin Wang 3, 4, 5, 6, 7 , Bangjiao Ye 3, 4, 5, 8 , Zhengping Fu 1, 2, 3, 4, 5 , Yalin Lu 1, 2, 3, 4, 5
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2020-06-12 , DOI: 10.1039/d0ta03411h Huan Liu 1, 2, 3, 4, 5 , Xiaoning Li 3, 4, 5, 6, 7 , Cailing Peng 1, 2, 3, 4, 5 , Liuyang Zhu 1, 2, 3, 4, 5 , Yuanxi Zhang 1, 2, 3, 4, 5 , Huiru Cheng 3, 4, 5, 8 , Jiameng Cui 1, 2, 3, 4, 5 , Qingmei Wu 1, 2, 3, 4, 5 , Yingying Zhang 1, 2, 3, 4, 5 , Zezhi Chen 1, 2, 3, 4, 5 , Wei Zou 1, 2, 3, 4, 5 , Wen Gu 1, 2, 3, 4, 5 , Haoliang Huang 3, 4, 5, 6, 7 , Jianlin Wang 3, 4, 5, 6, 7 , Bangjiao Ye 3, 4, 5, 8 , Zhengping Fu 1, 2, 3, 4, 5 , Yalin Lu 1, 2, 3, 4, 5
Affiliation
|
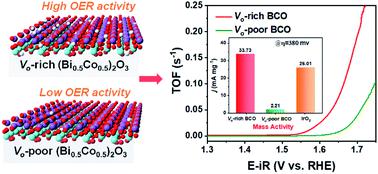
The catalytic activity for the oxygen evolution reaction (OER) in electrocatalytic water splitting strongly depends on the adsorption energy of intermediates. For the generally proposed adsorbate evolution route, the universal scaling relation between the adsorption energies of *OOH and *OH leads to an OER efficiency limitation based on the “volcano curve”. A possible solution to bypass the scaling relation is to avoid the formation of the *OOH intermediate in the OER with the participation of lattice oxygen from catalysts. In this work, the lattice oxygen in (Bi0.5Co0.5)2O3 is activated through adjusting the Fermi energy level and the strong overlap between Co 3d and O 2p, by means of increasing the oxygen vacancy concentration. Compared to oxygen-vacancy-poor (Bi0.5Co0.5)2O3, the oxygen-vacancy-rich (Bi0.5Co0.5)2O3 exhibits a significantly lower Tafel slope (43 mV dec−1), 15 times higher mass activity, 18 times higher turnover frequency, and excellent long-term stability in alkaline media, superior to those of the benchmark OER electrocatalyst IrO2. This work provides a feasible strategy to activate lattice oxygen with fast OER kinetics and puts forward the development of efficient and stable catalysts towards water oxidation.
中文翻译:

通过空位调制活化(Bi0.5Co0.5)2O3中的晶格氧,以实现有效的电化学水氧化
在电催化水分解过程中,氧气释放反应(OER)的催化活性强烈取决于中间体的吸附能。对于通常提出的吸附物逸出路线,* OOH和* OH的吸附能之间的通用比例关系导致基于“火山曲线”的OER效率限制。绕过比例关系的一种可能解决方案是避免在OER中形成* OOH中间体,而催化剂中的晶格氧会参与其中。在这项工作中,(Bi 0.5 Co 0.5)2 O 3中的晶格氧通过调节费米能级以及通过增加氧空位浓度来调节Co 3d和O 2p之间的强重叠来激活Sn。与贫氧空位(Bi 0.5 Co 0.5)2 O 3相比,富氧空位(Bi 0.5 Co 0.5)2 O 3的塔菲尔斜率明显更低(43 mV dec -1),质量高15倍。活性高,转换频率高18倍,在碱性介质中具有出色的长期稳定性,优于基准OER电催化剂IrO 2。这项工作提供了一种可行的策略,以快速的OER动力学活化晶格氧,并提出了开发有效,稳定的水氧化催化剂的方法。
更新日期:2020-07-07
中文翻译:

通过空位调制活化(Bi0.5Co0.5)2O3中的晶格氧,以实现有效的电化学水氧化
在电催化水分解过程中,氧气释放反应(OER)的催化活性强烈取决于中间体的吸附能。对于通常提出的吸附物逸出路线,* OOH和* OH的吸附能之间的通用比例关系导致基于“火山曲线”的OER效率限制。绕过比例关系的一种可能解决方案是避免在OER中形成* OOH中间体,而催化剂中的晶格氧会参与其中。在这项工作中,(Bi 0.5 Co 0.5)2 O 3中的晶格氧通过调节费米能级以及通过增加氧空位浓度来调节Co 3d和O 2p之间的强重叠来激活Sn。与贫氧空位(Bi 0.5 Co 0.5)2 O 3相比,富氧空位(Bi 0.5 Co 0.5)2 O 3的塔菲尔斜率明显更低(43 mV dec -1),质量高15倍。活性高,转换频率高18倍,在碱性介质中具有出色的长期稳定性,优于基准OER电催化剂IrO 2。这项工作提供了一种可行的策略,以快速的OER动力学活化晶格氧,并提出了开发有效,稳定的水氧化催化剂的方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号