当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Impact of Electrolyte Anions on the Adsorption of CO on Cu Electrodes
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-06-12 , DOI: 10.1021/acs.jpcc.0c04037 Vincent J. Ovalle 1 , Matthias M. Waegele 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-06-12 , DOI: 10.1021/acs.jpcc.0c04037 Vincent J. Ovalle 1 , Matthias M. Waegele 1
Affiliation
|
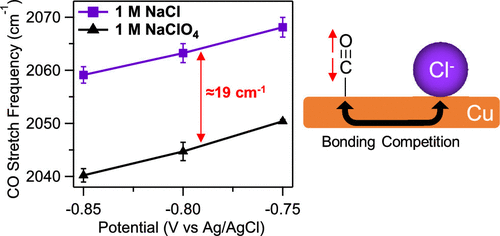
The electrocatalytic reduction of carbon dioxide to hydrocarbons and oxygenates on Cu electrodes proceeds through surface-adsorbed CO. The adsorption and desorption of this intermediate play a key role in determining the product selectivity of this electrocatalytic process. It is therefore critical to understand the molecular factors that determine the adsorption of CO on Cu electrodes. In prior studies, it was suggested that specifically adsorbing anions of the supporting electrolyte compete with CO for surface sites at low overpotentials. However, prior infrared (IR) spectroscopy of CO adsorption on Cu electrodes did not compare the relative CO coverages in the presence of different anions and was restricted to a narrow range of electrolyte concentrations (0.1 to 0.2 M). Therefore, the impact of anions on the adsorption of CO on Cu is not fully understood to date. Herein we systematically explored the adsorption and desorption of CO on polycrystalline Cu electrodes in the presence of specifically and nonspecifically adsorbing anions (Cl–, SO42–, ClO4–) at two different concentrations (10 mM and 1 M) of the corresponding sodium salts. With surface-enhanced IR absorption spectroscopy (SEIRAS), we monitored the C≡O stretch band of atop-bound CO (COatop) and an infrared band of the hydration shells of interfacial anions as a function of electrode potential. We found that at an electrolyte concentration of 10 mM, the adsorption and desorption of COatop are virtually independent of the identity of the anions. In contrast, at an electrolyte concentration of 1 M, the COatop coverage is significantly impacted by the electrolyte anions. The saturation coverages of COatop are lower in the 1 M electrolytes compared with those in the 10 mM electrolytes. The magnitude and mechanism of the modulation depend on the identity of the anions. Weakly and nonspecifically adsorbing anions (SO42–, ClO4–) limit the COatop saturation coverage by blocking a fraction of CO adsorption sites. However, their site-blocking ability depends on the CO coverage, as evidenced by the hysteresis in the CO adsorption/desorption profiles, which likely originates from a reversible CO-induced surface reconstruction. Chloride ions, which can specifically adsorb on Cu electrodes, lower the CO coverage by modulating the CO adsorption energy. This modulation manifests itself in (1) a distinctly higher C≡O stretch frequency in the presence of this anion relative to that measured in the other electrolytes and (2) the absence of the hysteresis in the adsorption/desorption profiles. Our study highlights the intricate interplay between anions and surface-adsorbed CO at the Cu electrode/electrolyte interface.
中文翻译:

电解质阴离子对一氧化碳在铜电极上吸附的影响
铜电极上的二氧化碳通过表面吸附的CO电催化还原为碳氢化合物和含氧化合物。该中间体的吸附和解吸在确定此电催化过程的产物选择性中起关键作用。因此,了解决定CO在Cu电极上吸附的分子因素至关重要。在先前的研究中,有人建议在低过电势下,专门吸附辅助电解质的阴离子与CO竞争表面部位。但是,现有的红外(IR)光谱分析表明,在不同的阴离子存在下,CO吸附在Cu电极上无法比较相对的CO覆盖率,并且只能在较窄的电解质浓度范围内(0.1到0.2 M)。因此,迄今为止,还没有完全了解阴离子对CO在Cu上的吸附的影响。在此,我们系统地研究了在存在特异性和非特异性吸附阴离子(Cl-,SO 4 2-,CLO 4 - )在相应的钠盐的两种不同浓度(10 mM和1 M)。使用表面增强的红外吸收光谱(SEIRAS),我们监测了顶部结合的CO的C≡O拉伸带(CO atop)和界面阴离子的水合壳的红外带随电极电位的变化。我们发现,在电解质浓度为10 mM时,顶部的CO的吸附和解吸实际上与阴离子的身份无关。相反,在电解质浓度为1 M时,电解质阴离子会显着影响CO顶部的覆盖率。CO的饱和覆盖度的顶上与10 mM电解质相比,1 M电解质的含量更低。调制的幅度和机理取决于阴离子的身份。弱和非特异性吸附的阴离子(SO 4 2-,CLO 4 - )限制CO顶上通过阻止一部分CO吸附位点实现饱和覆盖。但是,它们的位阻能力取决于CO的覆盖范围,这由CO吸附/解吸曲线中的滞后现象所证明,这很可能源自可逆的CO诱导的表面重建。可以专门吸附在Cu电极上的氯离子通过调节CO吸附能来降低CO覆盖率。这种调节作用表现为:(1)在该阴离子存在下相对于其他电解质所测得的C theO拉伸频率明显更高;(2)在吸附/解吸曲线中没有滞后现象。我们的研究强调了在铜电极/电解质界面处阴离子与表面吸附的CO之间的复杂相互作用。
更新日期:2020-07-09
中文翻译:

电解质阴离子对一氧化碳在铜电极上吸附的影响
铜电极上的二氧化碳通过表面吸附的CO电催化还原为碳氢化合物和含氧化合物。该中间体的吸附和解吸在确定此电催化过程的产物选择性中起关键作用。因此,了解决定CO在Cu电极上吸附的分子因素至关重要。在先前的研究中,有人建议在低过电势下,专门吸附辅助电解质的阴离子与CO竞争表面部位。但是,现有的红外(IR)光谱分析表明,在不同的阴离子存在下,CO吸附在Cu电极上无法比较相对的CO覆盖率,并且只能在较窄的电解质浓度范围内(0.1到0.2 M)。因此,迄今为止,还没有完全了解阴离子对CO在Cu上的吸附的影响。在此,我们系统地研究了在存在特异性和非特异性吸附阴离子(Cl-,SO 4 2-,CLO 4 - )在相应的钠盐的两种不同浓度(10 mM和1 M)。使用表面增强的红外吸收光谱(SEIRAS),我们监测了顶部结合的CO的C≡O拉伸带(CO atop)和界面阴离子的水合壳的红外带随电极电位的变化。我们发现,在电解质浓度为10 mM时,顶部的CO的吸附和解吸实际上与阴离子的身份无关。相反,在电解质浓度为1 M时,电解质阴离子会显着影响CO顶部的覆盖率。CO的饱和覆盖度的顶上与10 mM电解质相比,1 M电解质的含量更低。调制的幅度和机理取决于阴离子的身份。弱和非特异性吸附的阴离子(SO 4 2-,CLO 4 - )限制CO顶上通过阻止一部分CO吸附位点实现饱和覆盖。但是,它们的位阻能力取决于CO的覆盖范围,这由CO吸附/解吸曲线中的滞后现象所证明,这很可能源自可逆的CO诱导的表面重建。可以专门吸附在Cu电极上的氯离子通过调节CO吸附能来降低CO覆盖率。这种调节作用表现为:(1)在该阴离子存在下相对于其他电解质所测得的C theO拉伸频率明显更高;(2)在吸附/解吸曲线中没有滞后现象。我们的研究强调了在铜电极/电解质界面处阴离子与表面吸附的CO之间的复杂相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号