当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Achiral CpxIr(III)/Chiral Carboxylic Acid Catalyzed Enantioselective C–H Amidation of Ferrocenes under Mild Conditions
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-06-12 , DOI: 10.1021/acscatal.0c02049 Lei Liu 1 , Hong Song 1 , Yan-Hua Liu 1 , Le-Song Wu 1 , Bing-Feng Shi 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-06-12 , DOI: 10.1021/acscatal.0c02049 Lei Liu 1 , Hong Song 1 , Yan-Hua Liu 1 , Le-Song Wu 1 , Bing-Feng Shi 1
Affiliation
|
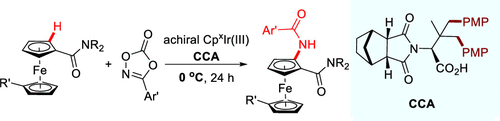
Ir(III)-catalyzed enantioselective C–H activation generally relies on the combination of chiral Ir(III) cyclopentadienyl complexes with chiral carboxylic acids to ensure high enantiocontrol. We report herein the achiral CpxIr(III)-catalyzed enantioselective C–H amidation of ferrocenes. Crucial to the high enantioselectivity is the use of a chiral carboxylic acid ligand derived from tert-leucine via Pd(II)-catalyzed γ-C(sp3)–H arylation and a sterically more hindered CpxIr(III) catalyst. This reaction proceeds smoothly under very mild conditions (0 °C) and tolerates a wide range of ferrocene carboxamides and dioxazolones, providing the amidation products in good yields with high enantioselectivity (up to 97.5:2.5 er). This protocol might open the way for achiral CpxIr(III)-catalyzed asymmetric C–H activation.
中文翻译:

手性Cp x Ir(III)/手性羧酸在温和条件下催化二茂铁的对映选择性C–H酰胺化
Ir(III)催化的对映选择性C–H活化通常依赖于手性Ir(III)环戊二烯基配合物与手性羧酸的结合,以确保对映体的高度控制。我们在此报告了二茂铁的非手性Cp x Ir(III)催化的对映选择性CH酰胺化反应。高对映选择性的关键是使用叔亮氨酸通过Pd(II)催化的γ-C(sp 3)-H芳基化和空间位阻更大的Cp x衍生的手性羧酸配体Ir(III)催化剂。该反应可在非常温和的条件下(0°C)顺利进行,并能耐受各种二茂铁羧酰胺和二恶唑酮,从而以高收率和高对映选择性(高达97.5:2.5 er)提供酰胺化产物。该协议可能为非手性Cp x Ir(III)催化的不对称CH活化开辟道路。
更新日期:2020-07-02
中文翻译:

手性Cp x Ir(III)/手性羧酸在温和条件下催化二茂铁的对映选择性C–H酰胺化
Ir(III)催化的对映选择性C–H活化通常依赖于手性Ir(III)环戊二烯基配合物与手性羧酸的结合,以确保对映体的高度控制。我们在此报告了二茂铁的非手性Cp x Ir(III)催化的对映选择性CH酰胺化反应。高对映选择性的关键是使用叔亮氨酸通过Pd(II)催化的γ-C(sp 3)-H芳基化和空间位阻更大的Cp x衍生的手性羧酸配体Ir(III)催化剂。该反应可在非常温和的条件下(0°C)顺利进行,并能耐受各种二茂铁羧酰胺和二恶唑酮,从而以高收率和高对映选择性(高达97.5:2.5 er)提供酰胺化产物。该协议可能为非手性Cp x Ir(III)催化的不对称CH活化开辟道路。











































 京公网安备 11010802027423号
京公网安备 11010802027423号