当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Indazolone‐Assisted Sequential ortho‐Alkenylation‐Oxidative Aza‐Michael Addition of 1‐Arylindazolone Using Acrylates Under Ru(II) Catalysis
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-06-12 , DOI: 10.1002/ajoc.202000239 Chikkagundagal K. Mahesha 1 , Sanjay K. Mandal 2 , Rajeev Sakhuja 1
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-06-12 , DOI: 10.1002/ajoc.202000239 Chikkagundagal K. Mahesha 1 , Sanjay K. Mandal 2 , Rajeev Sakhuja 1
Affiliation
|
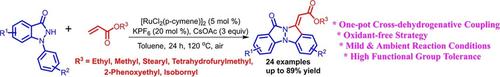
A one‐pot annulation of 1‐arylindazolones and acrylates is achieved through Ru(II)‐catalyzed sequential ortho‐alkenylation followed by oxidative intramolecular aza‐Michael addition reaction to deliver substituted indazolo[1,2‐a ]indazolylidenes in good‐to‐excellent yields. The strategy showcased high functional group tolerance and the directing group ability of indazolone moiety was established for Csp2‐H bond activation.
中文翻译:

在Ru(II)催化下,使用丙烯酸酯对吲哚酮辅助的1-芳基吲唑酮的顺序邻位烯基化氧化性氮杂-迈克尔加成反应
1- arylindazolones的单釜环酯和丙烯酸酯是通过钌实现(II) -催化的连续邻-alkenylation随后氧化分子内氮杂迈克尔加成反应以提供取代的吲唑并[1,2-一个良好-TO-] indazolylidenes优异的产量。该策略显示了较高的官能团耐受性,并确定了吲唑酮部分的定向基团能力可用于Csp 2 -H键激活。
更新日期:2020-08-08
中文翻译:

在Ru(II)催化下,使用丙烯酸酯对吲哚酮辅助的1-芳基吲唑酮的顺序邻位烯基化氧化性氮杂-迈克尔加成反应
1- arylindazolones的单釜环酯和丙烯酸酯是通过钌实现(II) -催化的连续邻-alkenylation随后氧化分子内氮杂迈克尔加成反应以提供取代的吲唑并[1,2-一个良好-TO-] indazolylidenes优异的产量。该策略显示了较高的官能团耐受性,并确定了吲唑酮部分的定向基团能力可用于Csp 2 -H键激活。











































 京公网安备 11010802027423号
京公网安备 11010802027423号