European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2020-06-12 , DOI: 10.1016/j.ejmech.2020.112273 Ziwen Zhang 1 , Jingli Min 1 , Mengdie Chen 1 , Xia Jiang 1 , Yingying Xu 1 , Huali Qin 2 , Wenjian Tang 1
|
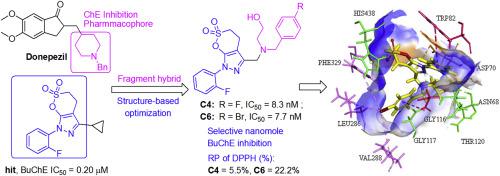
Structure-based optimization was conducted to improve the potency and selectivity of BuChE inhibitors with δ-sulfonolactone-fused pyrazole scaffold. By mimicking the hydrophobic interactions of donepezil at PAS, the introduction of a tertiary benzylamine at 5-position can significantly increase BuChE inhibitory activity. Compounds C4 and C6 were identified as high selective nanomolar BuChE inhibitors (IC50 = 8.3 and 7.7 nM, respectively), which exhibited mild antioxidant capacity, nontoxicity, lipophilicity and neuroprotective activity. Kinetic studies showed that BuChE inhibition of compound C6 was mixed-type against BuChE (Ki = 24 nM) and >2000-fold selectivity for BuChE over AChE. The proposed binding mode of new inhibitors was consistent with the results of structure–activity relationship analysis.
中文翻译:

δ-磺内酯基吡唑类化合物作为选择性BuChE抑制剂的结构优化。
进行了基于结构的优化,以提高BuChE抑制剂与δ-磺内酯融合的吡唑支架的效价和选择性。通过模仿多奈哌齐在PAS上的疏水相互作用,在5位引入叔苄胺可显着提高BuChE抑制活性。化合物C4和C6被确定为高选择性纳摩尔BuChE抑制剂(IC 50分别 为8.3和7.7 nM),具有轻度的抗氧化能力,无毒,亲脂性和神经保护活性。动力学研究表明,BuChE对化合物C6的抑制作用是针对BuChE(K i = 24 nM),BuChE的选择性是AChE的> 2000倍。所提出的新抑制剂的结合模式与结构-活性关系分析的结果一致。



























 京公网安备 11010802027423号
京公网安备 11010802027423号