Chemical Physics Letters ( IF 2.8 ) Pub Date : 2020-06-12 , DOI: 10.1016/j.cplett.2020.137697 Jian Lv , Qingshuang Wang , Jianxun Zhao , Wanqiang Liu , Peng Chen , Heng Liu
|
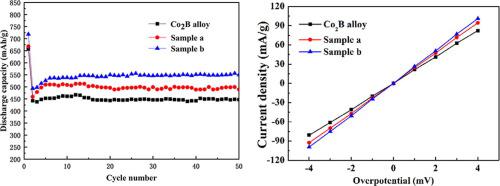
The Co2B hydrogen storage alloy is synthetized by high temperature solid phase process. Copper coated Co2B alloys are prepared by ball milling and element replacement reaction respectively. The structural features of Co2B alloys and copper coated alloys are investigated using XRD and SEM analysis. It can be found that the surface of the material obtained by the element displacement reaction is coated with more copper than the material obtained by mechanical alloying process. The electrochemical hydrogen storage performance of the copper coated alloy electrodes is measured at 303 K. At initial cycle, copper coated Co2B alloy electrodes exceed the raw Co2B alloy in discharge capacity. The alloy obtained by element replacement reaction has the highest capacity of 718.4 mAh/g, what’s more, the cycle life of copper coated Co2B alloy electrodes is higher than that of raw Co2B alloy. The capacity retention ratio and charge-transfer resistance (Rct) of the copper covered Co2B alloy electrodes decrease because of copper coating. The excellent electrochemical hydrogen storage performance of two copper coated Co2B alloy electrodes can be put down to the addition of copper, which increases the conductivity of the alloy electrode and reduce the area of the alloy electrode exposed to the electrolyte, slows down the oxidation of boron and its dissolution into the electrolyte. Copper coated on the surface of the alloy plays a favorable effect on electrochemical properties of Co2B alloy. The displacement reaction method is better than the ball-milling method in coating the surface of Co2B alloy to improve its electrochemical hydrogen storage performance.
中文翻译:

Co 2 B合金表面镀铜两种方法在电化学储氢性能改善上的差异
通过高温固相法合成Co 2 B储氢合金。铜包覆的Co 2 B合金分别通过球磨和元素置换反应制备。利用XRD和SEM分析研究了Co 2 B合金和镀铜合金的结构特征。可以发现,与通过机械合金化工艺获得的材料相比,通过元素置换反应获得的材料的表面涂覆了更多的铜。铜涂层合金电极的电化学储氢性能在303 K下测量。在初始循环中,铜涂层Co 2 B合金电极超过了原始Co 2B合金的放电容量。通过元素置换反应获得的合金的最高容量为718.4 mAh / g,而且镀铜的Co 2 B合金电极的循环寿命高于未加工的Co 2 B合金。覆铜的Co 2 B合金电极的容量保持率和电荷转移电阻(R ct)由于覆铜而降低。两种覆铜Co 2的优异电化学储氢性能可以降低B合金电极的含铜量,从而增加合金电极的电导率并减少暴露于电解质中的合金电极的面积,减慢硼的氧化及其在电解质中的溶解。涂覆在合金表面上的铜对Co 2 B合金的电化学性能具有有利的影响。置换反应法在球涂Co 2 B合金表面上优于球磨法,提高了电化学储氢性能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号