当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective oxo ligand functionalisation and substitution reactivity in an oxo/catecholate-bridged UIV/UIV Pacman complex
Chemical Science ( IF 7.6 ) Pub Date : 2020-06-12 , DOI: 10.1039/d0sc02297g Bradley E Cowie 1 , Iskander Douair 2 , Laurent Maron 2 , Jason B Love 1 , Polly L Arnold 1
Chemical Science ( IF 7.6 ) Pub Date : 2020-06-12 , DOI: 10.1039/d0sc02297g Bradley E Cowie 1 , Iskander Douair 2 , Laurent Maron 2 , Jason B Love 1 , Polly L Arnold 1
Affiliation
|
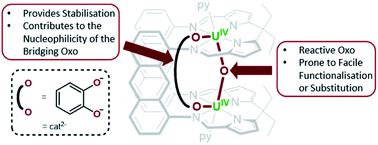
The oxo- and catecholate-bridged UIV/UIV Pacman complex [{(py)UIVOUIV(μ-O2C6H4)(py)}(LA)] A (LA = a macrocyclic “Pacman” ligand; anthracenylene hinge between N4-donor pockets, ethyl substituents on meso-carbon atom of each N4-donor pocket) featuring a bent UIV–O–UIV oxo bridge readily reacts with small molecule substrates to undergo either oxo-atom functionalisation or substitution. Complex A reacts with H2O or MeOH to afford [{(py)UIV(μ-OH)2UIV(μ-O2C6H4)(py)}(LA)] (1) and [{(py)UIV(μ-OH)(μ-OMe)UIV(μ-O2C6H4)(py)}(LA)] (2), respectively, in which the bridging oxo ligand in A is substituted for two bridging hydroxo ligands or one bridging hydroxo and one bridging methoxy ligand, respectively. Alternatively, A reacts with either 0.5 equiv. of S8 or 4 equiv. of Se to provide [{(py)UIV(μ-η2:η2-E2)UIV(μ-O2C6H4)(py)}(LA)] (E = S (3), Se (4)) respectively, in which the [E2]2− ion bridges the two UIV centres. To the best of our knowledge, complex A is the first example of either a d- or f-block bimetallic μ-oxo complex that activates elemental chalcogens. Complex A also reacts with XeF2 or 2 equiv. of Me3SiCl to provide [{(py)UIV(μ-X)2UIV(μ-O2C6H4)(py)}(LA)] (X = F (5), Cl (6)), in which the oxo ligand has been substituted for two bridging halido ligands. Reacting A with either XeF2 or Me3SiCl in the presence of O(Bcat)2 at room temperature forms [{(py)UIV(μ-X)(μ-OBcat)UIV(μ-O2C6H4)(py)}(LA)] (X = F (5A), Cl (6A)), which upon heating to 80 °C is converted to 5 and 6, respectively. In order to probe the importance of the bent UIV–O–UIV motif in A on the observed reactivity, the bis(boroxido)-UIV/UIV complex, [{(py)(pinBO)UIVOUIV(OBpin)(py)}(LA)] (B), featuring a linear UIV–O–UIV bond angle was treated with H2O and Me3SiCl. Complex B reacts with two equiv. of either H2O or Me3SiCl to provide [{(py)HOUIVOUIVOH(py)}(LA)] (7) and [{(py)ClUIVOUIVCl(py)}(LA)] (8), respectively, in which reactions occur preferentially at the boroxido ligands, with the μ-oxo ligand unchanged. The formal UIV oxidation state is retained in all of the products 1–8, and selective reactions at the bridging oxo ligand in A is facilitated by: (1) its highly nucleophilic character which is a result of a non-linear UIV–O–UIV bond angle causing an increase in U–O bond covalency and localisation of the lone pairs of electrons on the μ-oxo group, and (2) the presence of the bridging catecholate ligand, which destabilises a linear oxo-bridging geometry and stabilises the resulting products.
中文翻译:

氧代/儿茶酚酸酯桥联 UIV/UIV Pacman 复合物中的选择性氧代配体功能化和取代反应性
氧代和儿茶酸盐桥联的 U IV /U IV Pacman 复合物 [{(py)U IV OU IV (μ-O 2 C 6 H 4 )(py)}(L A )] A (L A = 大环“ Pacman”配体;在 N 4 -供体口袋之间的蒽铰链,每个 N 4 -供体口袋的中间碳原子上的乙基取代基)具有弯曲的 U IV –O–U IV氧代桥,很容易与小分子底物反应以进行氧代-原子功能化或取代。配合物A与 H 2反应O 或 MeOH 提供 [{(py)U IV (μ-OH) 2 U IV (μ-O 2 C 6 H 4 )(py)}(LA ) ] ( 1 ) 和 [{(py)U IV (μ-OH)(μ-OMe)U IV (μ-O 2 C 6 H 4 )(py)}(L A )] ( 2 ),其中A中的桥连氧代配体取代了两个桥连羟基配体或一种桥接羟基和一种桥接甲氧基配体。或者,A与 0.5 equiv 发生反应。S 8或 4 等值。Se 提供 [{(py)UIV (μ-η 2 :η 2 -E 2 )U IV (μ-O 2 C 6 H 4 )(py)}(L A )] (E = S ( 3 ), Se ( 4 )) 分别在[E 2 ] 2−离子桥接两个 U IV中心。据我们所知,络合物A是第一个激活元素硫属元素的 d 或 f 嵌段双金属 μ-氧络合物的例子。复合物A还与 XeF 2或 2 equiv 发生反应。Me 3 SiCl 提供 [{(py)U IV (μ-X)2 U IV (μ-O 2 C 6 H 4 )(py)}(L A )] (X = F ( 5 ), Cl ( 6 )),其中氧代配体已被两个桥接卤代配体取代。在室温下,在 O(Bcat) 2 存在下,A 与 XeF 2 或 Me 3 SiCl反应形成[ { ( py ) U IV (μ-X)(μ-OBcat)U IV (μ-O 2 C 6 H 4 )(py)}(L A )] (X = F ( 5A ), Cl ( 6A )),加热到 80 °C 时转化为分别为5和6。为了探究 A 中弯曲的 U IV -O -U IV基序对观察到的反应性的重要性,双(硼氧)-U IV /U IV复合物 [{(py)(pinBO)U IV OU IV ( OBpin)(py)}(L A )] ( B ),具有线性 U IV –O–U IV键角,用 H 2 O 和 Me 3 SiCl 处理。复合物B与两个当量反应。H 2 O 或 Me 3 SiCl 提供 [{(py)HOU IV OU IVOH(py)}( LA )] ( 7 ) 和 [{(py)ClU IV OU IV Cl (py)}( LA )] ( 8 ),其中反应优先发生在硼氧化物配体上,与μ-氧代配体不变。正式的 U IV氧化态保留在所有产物1-8中, A中的桥接氧代配体的选择性反应通过以下方式促进:(1)其高度亲核特性,这是非线性 U IV的结果-欧四键角导致 U-O 键共价增加和孤对电子在 μ-氧代基团上的定位,以及 (2) 桥接邻苯二甲酸盐配体的存在,它使线性氧代桥接几何结构不稳定并稳定所得产品。
更新日期:2020-07-15
中文翻译:

氧代/儿茶酚酸酯桥联 UIV/UIV Pacman 复合物中的选择性氧代配体功能化和取代反应性
氧代和儿茶酸盐桥联的 U IV /U IV Pacman 复合物 [{(py)U IV OU IV (μ-O 2 C 6 H 4 )(py)}(L A )] A (L A = 大环“ Pacman”配体;在 N 4 -供体口袋之间的蒽铰链,每个 N 4 -供体口袋的中间碳原子上的乙基取代基)具有弯曲的 U IV –O–U IV氧代桥,很容易与小分子底物反应以进行氧代-原子功能化或取代。配合物A与 H 2反应O 或 MeOH 提供 [{(py)U IV (μ-OH) 2 U IV (μ-O 2 C 6 H 4 )(py)}(LA ) ] ( 1 ) 和 [{(py)U IV (μ-OH)(μ-OMe)U IV (μ-O 2 C 6 H 4 )(py)}(L A )] ( 2 ),其中A中的桥连氧代配体取代了两个桥连羟基配体或一种桥接羟基和一种桥接甲氧基配体。或者,A与 0.5 equiv 发生反应。S 8或 4 等值。Se 提供 [{(py)UIV (μ-η 2 :η 2 -E 2 )U IV (μ-O 2 C 6 H 4 )(py)}(L A )] (E = S ( 3 ), Se ( 4 )) 分别在[E 2 ] 2−离子桥接两个 U IV中心。据我们所知,络合物A是第一个激活元素硫属元素的 d 或 f 嵌段双金属 μ-氧络合物的例子。复合物A还与 XeF 2或 2 equiv 发生反应。Me 3 SiCl 提供 [{(py)U IV (μ-X)2 U IV (μ-O 2 C 6 H 4 )(py)}(L A )] (X = F ( 5 ), Cl ( 6 )),其中氧代配体已被两个桥接卤代配体取代。在室温下,在 O(Bcat) 2 存在下,A 与 XeF 2 或 Me 3 SiCl反应形成[ { ( py ) U IV (μ-X)(μ-OBcat)U IV (μ-O 2 C 6 H 4 )(py)}(L A )] (X = F ( 5A ), Cl ( 6A )),加热到 80 °C 时转化为分别为5和6。为了探究 A 中弯曲的 U IV -O -U IV基序对观察到的反应性的重要性,双(硼氧)-U IV /U IV复合物 [{(py)(pinBO)U IV OU IV ( OBpin)(py)}(L A )] ( B ),具有线性 U IV –O–U IV键角,用 H 2 O 和 Me 3 SiCl 处理。复合物B与两个当量反应。H 2 O 或 Me 3 SiCl 提供 [{(py)HOU IV OU IVOH(py)}( LA )] ( 7 ) 和 [{(py)ClU IV OU IV Cl (py)}( LA )] ( 8 ),其中反应优先发生在硼氧化物配体上,与μ-氧代配体不变。正式的 U IV氧化态保留在所有产物1-8中, A中的桥接氧代配体的选择性反应通过以下方式促进:(1)其高度亲核特性,这是非线性 U IV的结果-欧四键角导致 U-O 键共价增加和孤对电子在 μ-氧代基团上的定位,以及 (2) 桥接邻苯二甲酸盐配体的存在,它使线性氧代桥接几何结构不稳定并稳定所得产品。











































 京公网安备 11010802027423号
京公网安备 11010802027423号