当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Deep Dive into DNA Base Pairing Interactions Under Water.
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-06-11 , DOI: 10.1021/acs.jpcb.0c03069 Rongpeng Li , Chi H. Mak
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-06-11 , DOI: 10.1021/acs.jpcb.0c03069 Rongpeng Li , Chi H. Mak
|
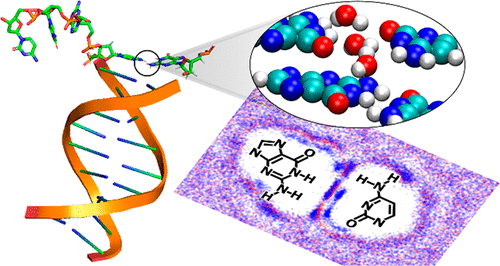
Base pairing plays a pivotal role in DNA functions and replication fidelity. But while the complementarity between Watson–Crick matched bases is generally believed to arise from the different number of hydrogen bonds in G|C pairs versus A|T, the energetics of these interactions are heavily renormalized by the aqueous solvent. Employing large-scale Monte Carlo simulations, we have extracted the solvent contribution to the free energy for canonical and some noncanonical and stacked base pairs. For all of them, the solvent’s contribution to the base pairing free energy is exclusively destabilizing. While the direct hydrogen bonding interactions in the G|C pair is much stronger than A|T, the thermodynamic resistance produced by the solvent also pushes back much stronger against G|C compared to A|T, generating an only ∼1 kcal/mol free energy difference between them. We have profiled the density of water molecules in the solvent adjacent to the bases and observed a “freezing” behavior where waters are recruited into the gap between the bases to compensate for the unsatisfied hydrogen bonds between them. A very small number of water molecules that are associated with the Watson–Crick donor/acceptor atoms turn out to be responsible for the majority of the solvent’s thermodynamic resistance to base pairing. The absence or presence of these near-field waters can be used to enhance fidelity during DNA replication.
中文翻译:

深入探讨水下DNA碱基配对的相互作用。
碱基配对在DNA功能和复制保真度中起关键作用。但是,尽管一般认为Watson-Crick匹配碱基之间的互补性是由于G | C对中相对于A | T的氢键数目不同而引起的,但这些相互作用的能量被水性溶剂重新规范化了。利用大规模的蒙特卡洛模拟,我们提取了规范对和一些非规范与堆叠碱基对的溶剂对自由能的贡献。对于所有这些溶剂,溶剂对碱基配对自由能的贡献完全是不稳定的。尽管G | C对中的直接氢键相互作用比A | T强得多,但与A | T相比,溶剂产生的对G | C的热力学阻力也要强得多,它们之间仅产生约1 kcal / mol的自由能差。我们分析了与碱相邻的溶剂中水分子的密度,并观察到“冻结”行为,其中水被吸收到碱之间的缝隙中,以补偿它们之间不满意的氢键。事实证明,与沃森克里克供体/受体原子相关的极少量水分子是造成溶剂对碱基配对的热力学抵抗力的主要来源。这些近场水的不存在或存在可以用来增强DNA复制过程中的保真度。我们分析了与碱相邻的溶剂中水分子的密度,并观察到“冻结”行为,其中水被吸收到碱之间的缝隙中,以补偿它们之间不满意的氢键。事实证明,与沃森克里克供体/受体原子相关的极少量水分子是造成溶剂对碱基配对的热力学抵抗力的主要来源。这些近场水的不存在或存在可以用来增强DNA复制过程中的保真度。我们分析了与碱相邻的溶剂中水分子的密度,并观察到“冻结”行为,其中水被吸收到碱之间的缝隙中,以补偿它们之间不满意的氢键。事实证明,与沃森克里克供体/受体原子相关的极少量水分子是造成溶剂对碱基配对的热力学抵抗力的主要来源。这些近场水的不存在或存在可以用来增强DNA复制过程中的保真度。
更新日期:2020-07-09
中文翻译:

深入探讨水下DNA碱基配对的相互作用。
碱基配对在DNA功能和复制保真度中起关键作用。但是,尽管一般认为Watson-Crick匹配碱基之间的互补性是由于G | C对中相对于A | T的氢键数目不同而引起的,但这些相互作用的能量被水性溶剂重新规范化了。利用大规模的蒙特卡洛模拟,我们提取了规范对和一些非规范与堆叠碱基对的溶剂对自由能的贡献。对于所有这些溶剂,溶剂对碱基配对自由能的贡献完全是不稳定的。尽管G | C对中的直接氢键相互作用比A | T强得多,但与A | T相比,溶剂产生的对G | C的热力学阻力也要强得多,它们之间仅产生约1 kcal / mol的自由能差。我们分析了与碱相邻的溶剂中水分子的密度,并观察到“冻结”行为,其中水被吸收到碱之间的缝隙中,以补偿它们之间不满意的氢键。事实证明,与沃森克里克供体/受体原子相关的极少量水分子是造成溶剂对碱基配对的热力学抵抗力的主要来源。这些近场水的不存在或存在可以用来增强DNA复制过程中的保真度。我们分析了与碱相邻的溶剂中水分子的密度,并观察到“冻结”行为,其中水被吸收到碱之间的缝隙中,以补偿它们之间不满意的氢键。事实证明,与沃森克里克供体/受体原子相关的极少量水分子是造成溶剂对碱基配对的热力学抵抗力的主要来源。这些近场水的不存在或存在可以用来增强DNA复制过程中的保真度。我们分析了与碱相邻的溶剂中水分子的密度,并观察到“冻结”行为,其中水被吸收到碱之间的缝隙中,以补偿它们之间不满意的氢键。事实证明,与沃森克里克供体/受体原子相关的极少量水分子是造成溶剂对碱基配对的热力学抵抗力的主要来源。这些近场水的不存在或存在可以用来增强DNA复制过程中的保真度。









































 京公网安备 11010802027423号
京公网安备 11010802027423号