当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Visible Light-Driven C4-Selective Alkylation of Pyridinium Derivatives with Alkyl Bromides
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-06-12 , DOI: 10.1021/jacs.0c04499 Sungwoo Jung 1, 2 , Sanghoon Shin 1, 2 , Seongjin Park 1, 2 , Sungwoo Hong 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-06-12 , DOI: 10.1021/jacs.0c04499 Sungwoo Jung 1, 2 , Sanghoon Shin 1, 2 , Seongjin Park 1, 2 , Sungwoo Hong 1, 2
Affiliation
|
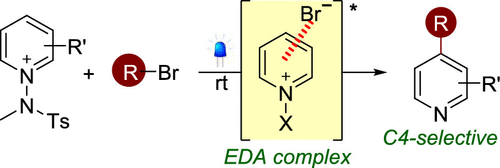
Reported herein is a general strategy for the photochemical cross-coupling between N-amidopyridinium salts and various alkyl bro-mides under photocatalyst-free conditions, granting facile access to various C4-alkylated pyridines. This approach exploits the intri-guing photochemical activity of electron donor-acceptor (EDA) complexes between N-amidopyridinium salts and bromide, which provides a photoactive handle capable of generating silyl radicals and driving the alkylation process. The robustness of this protocol was further demonstrated by the late-stage functionalization of complex compounds under mild and metal-free conditions.
中文翻译:

吡啶鎓衍生物与烷基溴的可见光驱动 C4 选择性烷基化
本文报道了在无光催化剂条件下 N-酰氨基吡啶鎓盐和各种烷基溴之间的光化学交叉偶联的一般策略,可以轻松获得各种 C4-烷基化吡啶。这种方法利用了 N-酰氨基吡啶鎓盐和溴化物之间的电子供体 - 受体 (EDA) 复合物的有趣光化学活性,它提供了能够产生甲硅烷基自由基并驱动烷基化过程的光活性处理。在温和和无金属条件下复杂化合物的后期功能化进一步证明了该协议的稳健性。
更新日期:2020-06-12
中文翻译:

吡啶鎓衍生物与烷基溴的可见光驱动 C4 选择性烷基化
本文报道了在无光催化剂条件下 N-酰氨基吡啶鎓盐和各种烷基溴之间的光化学交叉偶联的一般策略,可以轻松获得各种 C4-烷基化吡啶。这种方法利用了 N-酰氨基吡啶鎓盐和溴化物之间的电子供体 - 受体 (EDA) 复合物的有趣光化学活性,它提供了能够产生甲硅烷基自由基并驱动烷基化过程的光活性处理。在温和和无金属条件下复杂化合物的后期功能化进一步证明了该协议的稳健性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号