当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Comparative Dynamics and Functional Mechanisms of the CYP17A1 Tunnels Regulated by Ligand Binding.
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-06-12 , DOI: 10.1021/acs.jcim.0c00447 Fei Xiao 1 , Xingyu Song 1 , Peiyi Tian 1 , Mi Gan 1 , Gennady M Verkhivker 2, 3 , Guang Hu 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-06-12 , DOI: 10.1021/acs.jcim.0c00447 Fei Xiao 1 , Xingyu Song 1 , Peiyi Tian 1 , Mi Gan 1 , Gennady M Verkhivker 2, 3 , Guang Hu 1
Affiliation
|
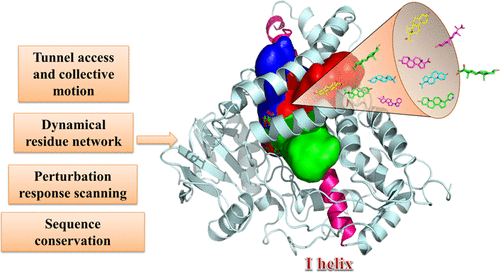
As an important member of cytochrome P450 (CYP) enzymes, CYP17A1 is a dual-function monooxygenase with a critical role in the synthesis of many human steroid hormones, making it an attractive therapeutic target. The emerging structural information about CYP17A1 and the growing number of inhibitors for these enzymes call for a systematic strategy to delineate and classify mechanisms of ligand transport through tunnels that control catalytic activity. In this work, we applied an integrated computational strategy to different CYP17A1 systems with a panel of ligands to systematically study at the atomic level the mechanism of ligand-binding and tunneling dynamics. Atomistic simulations and binding free energy computations identify the dynamics of dominant tunnels and characterize energetic properties of critical residues responsible for ligand binding. The common transporting pathways including S, 3, and 2c tunnels were identified in CYP17A1 binding systems, while the 2c tunnel is a newly formed pathway upon ligand binding. We employed and integrated several computational approaches including the analysis of functional motions and sequence conservation, atomistic modeling of dynamic residue interaction networks, and perturbation response scanning analysis to dissect ligand tunneling mechanisms. The results revealed the hinge-binding and sliding motions as main functional modes of the tunnel dynamic, and a group of mediating residues as key regulators of tunnel conformational dynamics and allosteric communications. We have also examined and quantified the mutational effects on the tunnel composition, conformational dynamics, and long-range allosteric behavior. The results of this investigation are fully consistent with the experimental data, providing novel rationale to the experiments and offering valuable insights into the relationships between the structure and function of the channel networks and a robust atomistic model of activation mechanisms and allosteric interactions in CYP enzymes.
中文翻译:

配体结合调控的 CYP17A1 隧道的比较动力学和功能机制。
作为细胞色素 P450 (CYP) 酶的重要成员,CYP17A1 是一种双功能单加氧酶,在许多人类类固醇激素的合成中起关键作用,使其成为一个有吸引力的治疗靶点。关于 CYP17A1 的新出现的结构信息和这些酶抑制剂数量的增加需要一种系统的策略来描述和分类配体通过控制催化活性的隧道转运的机制。在这项工作中,我们将集成计算策略应用于具有一组配体的不同 CYP17A1 系统,以在原子水平上系统地研究配体结合和隧道动力学的机制。原子模拟和结合自由能计算确定了主要隧道的动力学并表征了负责配体结合的关键残基的能量特性。在 CYP17A1 结合系统中鉴定了常见的转运途径,包括 S、3 和 2c 隧道,而 2c 隧道是配体结合后新形成的途径。我们采用并整合了几种计算方法,包括功能运动和序列守恒分析、动态残基相互作用网络的原子建模以及微扰响应扫描分析来剖析配体隧道机制。结果表明,铰链结合和滑动运动是隧道动力学的主要功能模式,而一组介导残基是隧道构象动力学和变构通信的关键调节因子。我们还检查并量化了突变对隧道组成、构象动力学和远程变构行为的影响。
更新日期:2020-07-27
中文翻译:

配体结合调控的 CYP17A1 隧道的比较动力学和功能机制。
作为细胞色素 P450 (CYP) 酶的重要成员,CYP17A1 是一种双功能单加氧酶,在许多人类类固醇激素的合成中起关键作用,使其成为一个有吸引力的治疗靶点。关于 CYP17A1 的新出现的结构信息和这些酶抑制剂数量的增加需要一种系统的策略来描述和分类配体通过控制催化活性的隧道转运的机制。在这项工作中,我们将集成计算策略应用于具有一组配体的不同 CYP17A1 系统,以在原子水平上系统地研究配体结合和隧道动力学的机制。原子模拟和结合自由能计算确定了主要隧道的动力学并表征了负责配体结合的关键残基的能量特性。在 CYP17A1 结合系统中鉴定了常见的转运途径,包括 S、3 和 2c 隧道,而 2c 隧道是配体结合后新形成的途径。我们采用并整合了几种计算方法,包括功能运动和序列守恒分析、动态残基相互作用网络的原子建模以及微扰响应扫描分析来剖析配体隧道机制。结果表明,铰链结合和滑动运动是隧道动力学的主要功能模式,而一组介导残基是隧道构象动力学和变构通信的关键调节因子。我们还检查并量化了突变对隧道组成、构象动力学和远程变构行为的影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号