当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tweaking the Charge Transfer: Bonding Analysis of Bismuth(III) Complexes with a Flexidentate Phosphane Ligand.
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-06-12 , DOI: 10.1021/acs.inorgchem.0c00734 Réka Mokrai 1 , Jamie Barrett 2 , David C Apperley 2 , Zoltán Benkő 1 , Dominikus Heift 2
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-06-12 , DOI: 10.1021/acs.inorgchem.0c00734 Réka Mokrai 1 , Jamie Barrett 2 , David C Apperley 2 , Zoltán Benkő 1 , Dominikus Heift 2
Affiliation
|
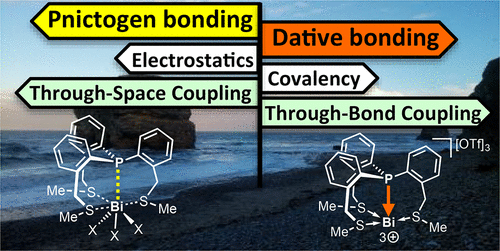
To account for the charge transfer and covalent character in bonding between P and Bi centers, the electronic structures of [P(C6H4-o-CH2SCH3)3BiCln](3–n)+ (n = 0–3) model species have been investigated computationally. On the basis of this survey a synthetic target compound with a dative P→Bi bond has been selected. Consecutively, the highly reactive bismuth cage [P(C6H4-o-CH2SCH3)3Bi]3+ has been accessed experimentally and characterized. Importantly, our experiments (single-crystal X-ray diffraction and solid-state NMR spectroscopy) and computations (NBO and AIM analysis) reveal that the P···Bi bonding in this trication can be described as a dative bond. Here we have shown that our accordion-like molecular framework allows for tuning of the interaction between P and Bi centers.
中文翻译:

调整电荷转移:铋 (III) 配合物与柔性膦配体的键合分析。
为了解释 P 和 Bi 中心之间键合中的电荷转移和共价特征,[P(C 6 H 4 - o -CH 2 SCH 3 ) 3 BiCl n ] (3– n )+ ( n = 0 –3) 模型物种已通过计算进行了研究。在此调查的基础上,选择了具有配位 P→Bi 键的合成目标化合物。随后,高活性铋笼[P(C 6 H 4 - o -CH 2 SCH 3 ) 3 Bi] 3+已通过实验获得并表征。重要的是,我们的实验(单晶X射线衍射和固态核磁共振波谱)和计算(NBO和AIM分析)表明,这种三阳离子中的P·Bi键可以描述为配价键。在这里,我们展示了我们的手风琴状分子框架可以调节 P 中心和 Bi 中心之间的相互作用。
更新日期:2020-07-06
中文翻译:

调整电荷转移:铋 (III) 配合物与柔性膦配体的键合分析。
为了解释 P 和 Bi 中心之间键合中的电荷转移和共价特征,[P(C 6 H 4 - o -CH 2 SCH 3 ) 3 BiCl n ] (3– n )+ ( n = 0 –3) 模型物种已通过计算进行了研究。在此调查的基础上,选择了具有配位 P→Bi 键的合成目标化合物。随后,高活性铋笼[P(C 6 H 4 - o -CH 2 SCH 3 ) 3 Bi] 3+已通过实验获得并表征。重要的是,我们的实验(单晶X射线衍射和固态核磁共振波谱)和计算(NBO和AIM分析)表明,这种三阳离子中的P·Bi键可以描述为配价键。在这里,我们展示了我们的手风琴状分子框架可以调节 P 中心和 Bi 中心之间的相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号