当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conformational Conversion Enhances Cellular Uptake of F Base Double-Strand-Conjugated Oligonucleotides.
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-06-12 , DOI: 10.1021/acs.analchem.0c00614 Huarong Bai 1 , Cheng Jin 1, 2 , Jianmei Zou 1 , Ruowen Wang 2 , Ting Fu 3 , Weihong Tan 1, 2
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-06-12 , DOI: 10.1021/acs.analchem.0c00614 Huarong Bai 1 , Cheng Jin 1, 2 , Jianmei Zou 1 , Ruowen Wang 2 , Ting Fu 3 , Weihong Tan 1, 2
Affiliation
|
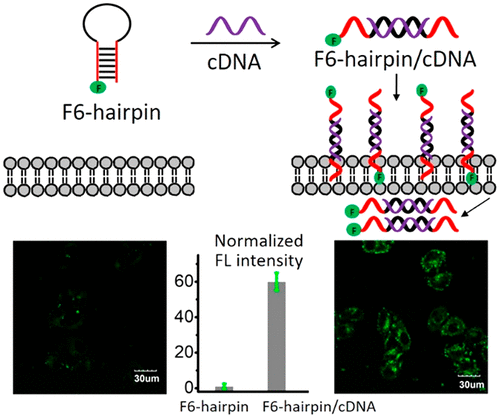
Artificial bases have emerged as a useful tool to expand genetic alphabets and biomedical applications of oligonucleotides. Herein, we reported that the conformation conversion enhances cellular uptake of hydrophobic 3,5-bis(trifluoromethyl)benzene (F) base double-strand-conjugated oligonucleotides. The formation of the F base double-strand caged the hydrophobic F base in the duplex strand, thus preventing F base from interacting with cells to some extent. However, upon conversion of F base double-strand-conjugated oligonucleotide to F base single-strand-conjugated oligonucleotide, F bases then were allowed to interact with cells by stronger hydrophobic interactions, followed by cellular uptake. The results were concluded as a pairing-induced cage effect of F base and have the potential for the construction of stimuli–responsive cellular uptake of functional nucleic acids.
中文翻译:

构象转化增强了F碱基双链缀合的寡核苷酸的细胞摄取。
人工碱基已经成为扩展遗传字母和寡核苷酸生物医学应用的有用工具。在本文中,我们报道了构象转化增强了疏水性3,5-双(三氟甲基)苯(F)碱基双链共轭寡核苷酸的细胞摄取。F碱基双链的形成将双链中的疏水性F碱基笼罩在一起,从而在某种程度上防止F碱基与细胞相互作用。然而,在将F碱基双链缀合的寡核苷酸转化为F碱基单链缀合的寡核苷酸后,然后使F碱基通过更强的疏水相互作用与细胞相互作用,随后被细胞摄取。
更新日期:2020-08-04
中文翻译:

构象转化增强了F碱基双链缀合的寡核苷酸的细胞摄取。
人工碱基已经成为扩展遗传字母和寡核苷酸生物医学应用的有用工具。在本文中,我们报道了构象转化增强了疏水性3,5-双(三氟甲基)苯(F)碱基双链共轭寡核苷酸的细胞摄取。F碱基双链的形成将双链中的疏水性F碱基笼罩在一起,从而在某种程度上防止F碱基与细胞相互作用。然而,在将F碱基双链缀合的寡核苷酸转化为F碱基单链缀合的寡核苷酸后,然后使F碱基通过更强的疏水相互作用与细胞相互作用,随后被细胞摄取。











































 京公网安备 11010802027423号
京公网安备 11010802027423号