当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Forging Odd-Membered Rings: Palladium-Catalyzed Asymmetric Cycloadditions of Trimethylenemethane.
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-06-11 , DOI: 10.1021/acs.accounts.0c00152 Barry M Trost 1 , Guillaume Mata 1, 2
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-06-11 , DOI: 10.1021/acs.accounts.0c00152 Barry M Trost 1 , Guillaume Mata 1, 2
Affiliation
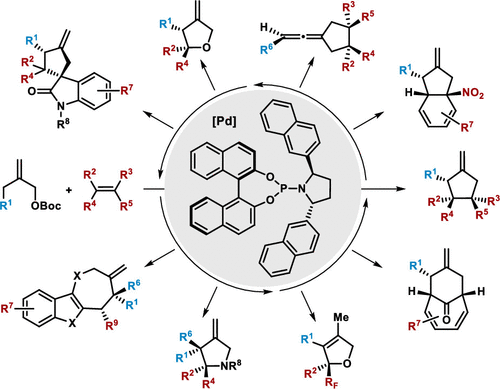
|
The catalytic asymmetric synthesis of complex molecules has been the focus of our research program for several decades because such strategies have significant utility for the construction of chiral building blocks for drug development as well as the total synthesis of natural products. Cycloaddition reactions are very powerful transformations in organic synthesis providing access to highly functionalized motifs from simple starting materials. In concert with this central interest, four decades ago, we reported the palladium-catalyzed trimethylenemethane (TMM) cycloaddition for forging odd-membered ring systems. In recent years, we focused our attention on the development of powerful ligand scaffolds which enable the preparation of valuable products with complete control of chemo-, regio-, diastereo-, and enantioselectivity, thereby addressing several limitations in the field of palladium-catalyzed asymmetric cycloadditions. The first section of this Account will outline the discovery of a new class of highly modular pyrrolidine-based phosphoramidite and diamidophosphite chiral ligands which facilitate [3 + 2] cycloadditions of TMM donors, opening a new area in asymmetric construction of five-membered rings.
中文翻译:

锻造奇数元环:钯催化的三亚甲基甲烷的不对称环加成反应。
几十年来,复杂分子的催化不对称合成一直是我们研究计划的重点,因为这种策略对于构建用于药物开发的手性结构单元以及天然产物的全合成具有重大效用。环加成反应是有机合成中非常有力的转化,可以从简单的起始原料获得高度官能化的基序。为了满足这一中心利益,四十年前,我们报道了钯催化的三亚甲基甲烷(TMM)环加成反应,用于锻造奇数环系统。近年来,我们将注意力集中在开发功能强大的配体支架上,该支架能够制备可完全控制化学,区域,非对映和对映选择性的有价值的产品,从而解决了钯催化的不对称环加成反应中的一些限制。该帐户的第一部分将概述发现一类新型的高度模块化的基于吡咯烷的亚磷酰胺和亚氨基二磷酸酯手性配体,它们有助于TMM供体的[3 + 2]环加成,为五元环的不对称结构开辟了一个新领域。
更新日期:2020-07-21
中文翻译:

锻造奇数元环:钯催化的三亚甲基甲烷的不对称环加成反应。
几十年来,复杂分子的催化不对称合成一直是我们研究计划的重点,因为这种策略对于构建用于药物开发的手性结构单元以及天然产物的全合成具有重大效用。环加成反应是有机合成中非常有力的转化,可以从简单的起始原料获得高度官能化的基序。为了满足这一中心利益,四十年前,我们报道了钯催化的三亚甲基甲烷(TMM)环加成反应,用于锻造奇数环系统。近年来,我们将注意力集中在开发功能强大的配体支架上,该支架能够制备可完全控制化学,区域,非对映和对映选择性的有价值的产品,从而解决了钯催化的不对称环加成反应中的一些限制。该帐户的第一部分将概述发现一类新型的高度模块化的基于吡咯烷的亚磷酰胺和亚氨基二磷酸酯手性配体,它们有助于TMM供体的[3 + 2]环加成,为五元环的不对称结构开辟了一个新领域。











































 京公网安备 11010802027423号
京公网安备 11010802027423号