当前位置:
X-MOL 学术
›
Stem Cells Transl. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Current status of mesenchymal stem cell therapy for immune/inflammatory lung disorders: Gleaning insights for possible use in COVID-19.
STEM CELLS Translational Medicine ( IF 5.4 ) Pub Date : 2020-06-11 , DOI: 10.1002/sctm.20-0186 B Linju Yen,Men-Luh Yen,Li-Tzu Wang,Ko-Jiunn Liu,Huey-Kang Sytwu
STEM CELLS Translational Medicine ( IF 5.4 ) Pub Date : 2020-06-11 , DOI: 10.1002/sctm.20-0186 B Linju Yen,Men-Luh Yen,Li-Tzu Wang,Ko-Jiunn Liu,Huey-Kang Sytwu
|
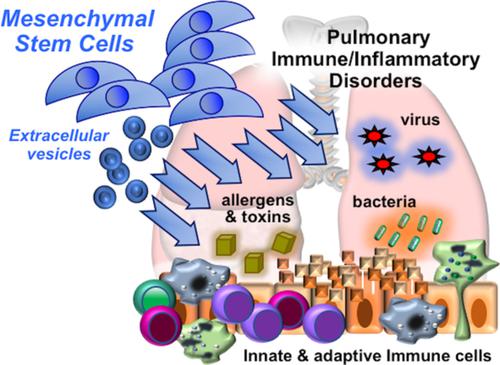
The broad immunomodulatory properties of human mesenchymal stem cells (MSCs) have allowed for wide application in regenerative medicine as well as immune/inflammatory diseases, including unmatched allogeneic use. The novel coronavirus disease COVID‐19 has unleashed a pandemic in record time accompanied by an alarming mortality rate mainly due to pulmonary injury and acute respiratory distress syndrome. Because there are no effective preventive or curative therapies currently, MSC therapy (MSCT) has emerged as a possible candidate despite the lack of preclinical data of MSCs for COVID‐19. Interestingly, MSCT preclinical data specifically on immune/inflammatory disorders of the lungs were among the earliest to be reported in 2003, with the first clinical use of MSCT for graft‐vs‐host disease reported in 2004. Since these first reports, preclinical data showing beneficial effects of MSC immunomodulation have accumulated substantially, and as a consequence, over a third of MSCT clinical trials now target immune/inflammatory diseases. There is much preclinical evidence for MSCT in noninfectious—including chronic obstructive pulmonary disease, asthma, and idiopathic pulmonary fibrosis—as well as infectious bacterial immune/inflammatory lung disorders, with data generally demonstrating therapeutic effects; however, for infectious viral pulmonary conditions, the preclinical evidence is more scarce with some inconsistent outcomes. In this article, we review the mechanistic evidence for clinical use of MSCs in pulmonary immune/inflammatory disorders, and survey the ongoing clinical trials—including for COVID‐19—of MSCT for these diseases, with some perspectives and comment on MSCT for COVID‐19.
中文翻译:

间充质干细胞治疗免疫/炎症性肺疾病的现状:可能在COVID-19中使用的见解。
人间充质干细胞(MSC)具有广泛的免疫调节特性,已广泛用于再生医学以及免疫/炎性疾病,包括无与伦比的同种异体用途。新型冠状病毒疾病COVID-19在创纪录的时间内引发了大流行,并伴随着惊人的死亡率,这主要是由于肺部损伤和急性呼吸窘迫综合征。由于目前尚无有效的预防或治疗方法,尽管缺乏针对COVID-19的MSC的临床前数据,但MSC治疗(MSCT)已成为一种可能的候选药物。有趣的是,最早于2003年报道了专门针对肺部免疫/炎性疾病的MSCT临床前数据,并于2004年首次报道了将MSCT用于移植物抗宿主疾病的首次临床应用。自从这些首次报道以来,显示MSC免疫调节有益作用的临床前数据已经大量积累,结果,超过三分之一的MSCT临床试验现在针对免疫/炎症性疾病。MSCT在非传染性疾病方面有许多临床前证据,包括慢性阻塞性肺疾病,哮喘和特发性肺纤维化,以及传染性细菌免疫/炎症性肺疾病,其数据通常证明具有治疗作用;然而,对于传染性病毒性肺部疾病,临床前证据更为稀少,且结果不一致。在本文中,我们回顾了MSC在肺部免疫/炎性疾病中的临床应用的机械证据,并调查了MSCT针对这些疾病进行的正在进行的临床试验(包括COVID-19),
更新日期:2020-06-11
中文翻译:

间充质干细胞治疗免疫/炎症性肺疾病的现状:可能在COVID-19中使用的见解。
人间充质干细胞(MSC)具有广泛的免疫调节特性,已广泛用于再生医学以及免疫/炎性疾病,包括无与伦比的同种异体用途。新型冠状病毒疾病COVID-19在创纪录的时间内引发了大流行,并伴随着惊人的死亡率,这主要是由于肺部损伤和急性呼吸窘迫综合征。由于目前尚无有效的预防或治疗方法,尽管缺乏针对COVID-19的MSC的临床前数据,但MSC治疗(MSCT)已成为一种可能的候选药物。有趣的是,最早于2003年报道了专门针对肺部免疫/炎性疾病的MSCT临床前数据,并于2004年首次报道了将MSCT用于移植物抗宿主疾病的首次临床应用。自从这些首次报道以来,显示MSC免疫调节有益作用的临床前数据已经大量积累,结果,超过三分之一的MSCT临床试验现在针对免疫/炎症性疾病。MSCT在非传染性疾病方面有许多临床前证据,包括慢性阻塞性肺疾病,哮喘和特发性肺纤维化,以及传染性细菌免疫/炎症性肺疾病,其数据通常证明具有治疗作用;然而,对于传染性病毒性肺部疾病,临床前证据更为稀少,且结果不一致。在本文中,我们回顾了MSC在肺部免疫/炎性疾病中的临床应用的机械证据,并调查了MSCT针对这些疾病进行的正在进行的临床试验(包括COVID-19),











































 京公网安备 11010802027423号
京公网安备 11010802027423号