当前位置:
X-MOL 学术
›
J. Chem. Thermodyn.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Determination of the vaporisation enthalpies for amino acid ionic liquids [C mim][Ala](n = 5, 6) and applications of the molar surface Gibbs energy
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.jct.2020.106164 Dawei Fang , Kunhao Liang , Chunxiao Song , Chengxuan Duan , Jie Wei
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.jct.2020.106164 Dawei Fang , Kunhao Liang , Chunxiao Song , Chengxuan Duan , Jie Wei
|
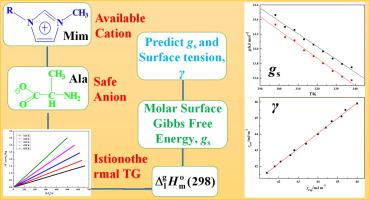
Abstract Amino acid ionic liquids (ILs) 1-alkyl-3-methylimidazolium alanine [Cnmim][Ala] (n = 5,6) were prepared and characterized. The enthalpy of vaporisation at the average temperature, Δ 1 g H m o (Tav), for the two ILs were determined by means of isothermogravimetrical analysis, and in terms of Δ 1 g C p , m o , the value of Δ 1 g H m o (Tav) can be adjusted to Δ 1 g H m o (2 9 8); then, the polarity, δμ, of the two ILs were estimated, in which the order of the δμ is in consistent with our experiences. In terms of molar surface Gibbs energy, gs, the traditional Eotvos equation was improved, the parameters of new Eotvos one have clear physical meanings: Its slope and intercept are the molar surface entropy, s, and the molar surface enthalpy, h, respectively. In addition, combining gs with Lorentz–Lorenz equation, an expression for predicting surface tension, γ, of the ILs was obtained, and the predicted values of γ are in good agreement with the experimental ones.
中文翻译:

氨基酸离子液体汽化焓的测定 [C mim][Ala](n = 5, 6) 和摩尔表面吉布斯能的应用
摘要 制备并表征了氨基酸离子液体 (IL) 1-烷基-3-甲基咪唑鎓丙氨酸 [Cnmim][Ala] (n = 5,6)。两种离子液体在平均温度下的汽化焓 Δ 1 g H mo (Tav) 是通过等温重量分析确定的,并以 Δ 1 g C p , mo 表示 Δ 1 g H mo 的值(Tav) 可调整为 Δ 1 g H mo (2 9 8);然后,估计两个IL的极性δμ,其中δμ的顺序与我们的经验一致。在摩尔表面吉布斯能 gs 方面,改进了传统的 Eotvos 方程,新 Eotvos 方程的参数具有明确的物理意义:其斜率和截距分别为摩尔表面熵 s 和摩尔表面焓 h。此外,结合 gs 与 Lorentz-Lorenz 方程,
更新日期:2020-11-01
中文翻译:

氨基酸离子液体汽化焓的测定 [C mim][Ala](n = 5, 6) 和摩尔表面吉布斯能的应用
摘要 制备并表征了氨基酸离子液体 (IL) 1-烷基-3-甲基咪唑鎓丙氨酸 [Cnmim][Ala] (n = 5,6)。两种离子液体在平均温度下的汽化焓 Δ 1 g H mo (Tav) 是通过等温重量分析确定的,并以 Δ 1 g C p , mo 表示 Δ 1 g H mo 的值(Tav) 可调整为 Δ 1 g H mo (2 9 8);然后,估计两个IL的极性δμ,其中δμ的顺序与我们的经验一致。在摩尔表面吉布斯能 gs 方面,改进了传统的 Eotvos 方程,新 Eotvos 方程的参数具有明确的物理意义:其斜率和截距分别为摩尔表面熵 s 和摩尔表面焓 h。此外,结合 gs 与 Lorentz-Lorenz 方程,











































 京公网安备 11010802027423号
京公网安备 11010802027423号