Molecular Therapy - Methods & Clinical Development ( IF 4.6 ) Pub Date : 2020-06-12 , DOI: 10.1016/j.omtm.2020.06.007 Szilárd Kiss 1 , Ruslan Grishanin 2 , Aivan Nguyen 2 , Romeo Rosario 2 , Judith S Greengard 2 , Julio Nieves 2 , Claire M Gelfman 2 , Mehdi Gasmi 2
|
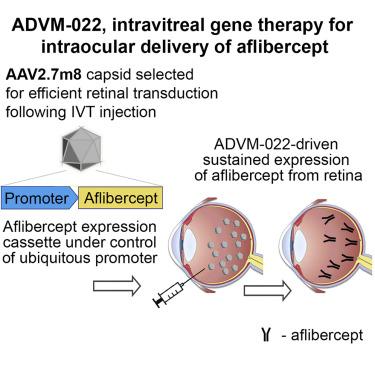
Several standard-of-care therapies for the treatment of retinal disease, including aflibercept, inhibit vascular endothelial growth factor (VEGFA). The main shortcoming of these therapies is potential undertreatment due to a lack of compliance resulting from the need for repeated injections. Gene therapy may provide sustained levels of anti-VEGFA proteins in the retina following a single injection. In this nonhuman primate study, we explored whether ADVM-022, a recombinant adeno-associated virus (AAV) vector designed to express aflibercept, could induce anti-VEGFA protein levels comparable with those observed following a single-bolus intravitreal (IVT) injection of the standard-of-care aflibercept recombinant protein. The results demonstrated that intraocular levels of aflibercept measured at 56 days after a single IVT injection of ADVM-022 were equivalent to those in the aflibercept recombinant protein-injected animals measured 21–32 days post-administration. ADVM-022-injected animals exhibited signs of an initial self-limiting inflammatory response, but overall all doses were well tolerated. ADVM-022 administration did not result in systemic exposure to aflibercept at any dose evaluated. These results demonstrated that a single IVT injection of ADVM-022 resulted in safe and efficacious aflibercept levels in the therapeutic range, suggesting the potential of a gene therapy approach for long-term treatment of retinal disease with anti-VEGF therapy.
中文翻译:

玻璃体给药ADVM-022(nAMD的潜在基因疗法)后,NHP中Aflibercept表达的分析。
几种用于治疗视网膜疾病的护理标准疗法,包括阿柏西普,可抑制血管内皮生长因子(VEGFA)。这些疗法的主要缺点是由于需要重复注射而导致缺乏依从性,从而可能导致治疗不足。单次注射后,基因疗法可在视网膜中提供持续水平的抗VEGFA蛋白。在这项非人类的灵长类动物研究中,我们探讨了ADVM-022(一种设计用于表达aflibercept的重组腺相关病毒(AAV)载体)是否可以诱导抗VEGFA蛋白水平,与单剂量玻璃体内注射IVT后观察到的水平相近。护理标准的阿柏西普重组蛋白。结果表明,单次IVT注射ADVM-022后56天测得的眼内aflibercept水平与注射后21-32天测得的aflibercept重组蛋白注射动物的眼内水平相同。注射ADVM-022的动物表现出初始的自限性炎症反应的迹象,但总体上所有剂量均耐受良好。在任何评估剂量下,ADVM-022给药均不会导致全身暴露于阿柏西普。这些结果表明,单次IVT注射ADVM-022可在治疗范围内产生安全有效的阿柏西普水平,这表明基因治疗方法可用于长期用抗VEGF治疗视网膜疾病。注射ADVM-022的动物表现出初始的自限性炎症反应的迹象,但总体上所有剂量均耐受良好。在任何评估剂量下,ADVM-022给药均不会导致全身暴露于阿柏西普。这些结果表明,单次IVT注射ADVM-022可在治疗范围内产生安全有效的阿柏西普水平,这表明基因治疗方法可长期用于抗VEGF治疗视网膜疾病。注射ADVM-022的动物表现出初始的自限性炎症反应的迹象,但总体上所有剂量均耐受良好。在任何评估剂量下,ADVM-022给药均不会导致全身暴露于阿柏西普。这些结果表明,单次IVT注射ADVM-022可在治疗范围内产生安全有效的阿柏西普水平,这表明基因治疗方法可用于长期用抗VEGF治疗视网膜疾病。











































 京公网安备 11010802027423号
京公网安备 11010802027423号