Journal of Catalysis ( IF 6.5 ) Pub Date : 2020-06-12 , DOI: 10.1016/j.jcat.2020.06.004 Jiaquan Wang , Jiajie Wu , Zhe-Ning Chen , Daheng Wen , Jiangbo Chen , Qingshu Zheng , Xin Xu , Tao Tu
|
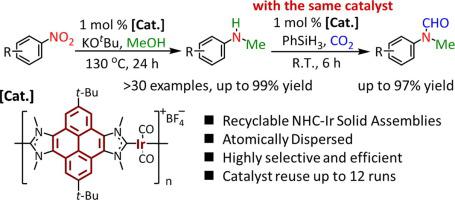
A series of N-heterocyclic carbene-iridium (NHC-Ir) coordination assemblies based on bis-pyrenoimidazolium salts are prepared, and shown to function as efficient solid molecular catalysts in selective mono-N-methylation of nitroarenes with methanol under mild conditions. The atomically dispersed active Ir(I) centers and the large π-conjugation rings endow the solid catalysts with an exceptionally high activity and selectivity for a broad substrate scope. Such solid NHC-Ir coordination assemblies are robust, which can be easily recovered and reused more than 10 runs without significant loss of their catalytic activity and selectivity. When combined with a subsequent formylation using the same solid catalysts under ambient conditions, this novel protocol can afford diverse formamides in excellent yields, further highlighting the applicability of the present solid catalysts for an efficient diversification of nitroarenes to a broad number of functional amines.
中文翻译:

原子分散的NHC-Ir固体组装体催化的甲醇对硝基芳烃的选择性单N-甲基化
制备了一系列基于双-吡咯并咪唑鎓盐的N-杂环卡宾-铱(NHC-Ir)配位组件,并显示出在选择性单N中有效的固体分子催化剂的作用在温和条件下用甲醇对硝基芳烃进行甲基化。原子分散的活性Ir(I)中心和大的π共轭环使固体催化剂在广泛的底物范围内具有异常高的活性和选择性。此类固体NHC-Ir配位组件坚固耐用,可以轻松回收并重复使用10多次以上,而不会显着降低其催化活性和选择性。当与随后在环境条件下使用相同固体催化剂的甲酰化反应相结合时,这种新颖的方法可以以优异的收率提供各种甲酰胺,进一步突显了本发明的固体催化剂对于硝基芳烃有效地多样化为多种功能胺的适用性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号