European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2020-06-12 , DOI: 10.1016/j.ejmech.2020.112557 Rostom Ahmed-Belkacem 1 , Priscila Sutto-Ortiz 2 , Mathis Guiraud 1 , Bruno Canard 2 , Jean-Jacques Vasseur 1 , Etienne Decroly 2 , Françoise Debart 1
|
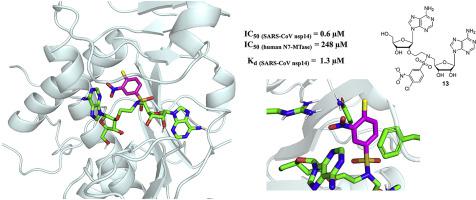
The spreading of new viruses is known to provoke global human health threat. The current COVID-19 pandemic caused by the recently emerged coronavirus SARS-CoV-2 is one significant and unfortunate example of what the world will have to face in the future with emerging viruses in absence of appropriate treatment. The discovery of potent and specific antiviral inhibitors and/or vaccines to fight these massive outbreaks is an urgent research priority. Enzymes involved in the capping pathway of viruses and more specifically RNA N7- or 2′O-methyltransferases (MTases) are now admitted as potential targets for antiviral chemotherapy. We designed bisubstrate inhibitors by mimicking the transition state of the 2′-O-methylation of the cap RNA in order to block viral 2′-O MTases. This work resulted in the synthesis of 16 adenine dinucleosides with both adenosines connected by various nitrogen-containing linkers. Unexpectedly, all the bisubstrate compounds were barely active against 2′-O MTases of several flaviviruses or SARS-CoV but surprisingly, seven of them showed efficient and specific inhibition against SARS-CoV N7-MTase (nsp14) in the micromolar to submicromolar range. The most active nsp14 inhibitor identified is as potent as but particularly more specific than the broad-spectrum MTase inhibitor, sinefungin. Molecular docking suggests that the inhibitor binds to a pocket formed by the S-adenosyl methionine (SAM) and cap RNA binding sites, conserved among SARS-CoV nsp14. These dinucleoside SAM analogs will serve as starting points for the development of next inhibitors for SARS-CoV-2 nsp14 N7-MTase.
中文翻译:

合成腺嘌呤二核苷SAM类似物,作为SARS-CoV nsp14 RNA帽鸟嘌呤-N7-甲基转移酶的特异性抑制剂。
已知新病毒的传播会引起全球人类健康威胁。由最近出现的冠状病毒SARS-CoV-2引起的当前COVID-19大流行是一个重要且不幸的例子,说明如果没有适当的治疗,将来世界将不得不面对新兴的病毒。对抗这些大规模暴发的有效和特异性抗病毒抑制剂和/或疫苗的发现是紧迫的研究重点。参与病毒的封盖途径,并且更具体RNA N7-或2的酶' ø -methyltransferases(MTases)现在被承认为抗病毒化学疗法的潜在靶标。我们通过模拟cap RNA 2'- O-甲基化的过渡态来设计双底物抑制剂,以阻断病毒2'- OMTases。这项工作导致了16种腺嘌呤二核苷的合成,两个腺苷都通过各种含氮接头连接。出乎意料的是,所有双底物化合物几乎都没有针对2'- O的活性几种黄病毒或SARS-CoV的MTase,但令人惊讶的是,其中的7种在微摩尔至亚微摩尔范围内显示出对SARS-CoV N7-MTase(nsp14)的有效和特异性抑制。鉴定出的最活跃的nsp14抑制剂与广谱MTase抑制剂西那芬净一样有效,但特异性更高。分子对接表明,该抑制剂与SARS-CoV nsp14中保守的S-腺苷甲硫氨酸(SAM)和cap RNA结合位点形成的口袋结合。这些二核苷SAM类似物将作为开发SARS-CoV-2 nsp14 N7-MTase抑制剂的起点。



























 京公网安备 11010802027423号
京公网安备 11010802027423号