当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical elucidation of the multi-functional synthetic methodology for switchable Ni(0)-catalyzed C–H allylations, alkenylations and dienylations with allenes
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2020-06-11 , DOI: 10.1039/d0cy00965b Yuxia Liu 1, 2, 3, 4, 5 , Kaifeng Wang 1, 2, 3, 4 , Baoping Ling 1, 2, 3, 4 , Guang Chen 1, 2, 3, 4 , Yulin Li 4, 6, 7, 8, 9 , Lingjun Liu 1, 2, 3, 4 , Siwei Bi 1, 2, 3, 4
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2020-06-11 , DOI: 10.1039/d0cy00965b Yuxia Liu 1, 2, 3, 4, 5 , Kaifeng Wang 1, 2, 3, 4 , Baoping Ling 1, 2, 3, 4 , Guang Chen 1, 2, 3, 4 , Yulin Li 4, 6, 7, 8, 9 , Lingjun Liu 1, 2, 3, 4 , Siwei Bi 1, 2, 3, 4
Affiliation
|
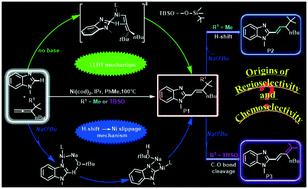
The Ni(0)-catalyzed coupling of benzimidazole with 1,1-disubstituted allenes represents a new strategy for achieving controllable C–H allylations, alkenylations and dienylations. To understand the detailed mechanisms and origins of the switchable selectivities, density functional theory (DFT) calculations were conducted. The results using a tBu-substituted allene demonstrate that the formation of the allylated product involves a Ni-catalyzed C–H activation mechanism through ligand-to-ligand-hydrogen transfer (LLHT) under base-free conditions. In contrast, a Ni/NaOtBu co-promoted C–H activation mechanism is newly proposed in the presence of NaOtBu, which is remarkably different from the previously reported literature. The novel mechanism emphasizes that NaOtBu abstracts the Ni-activated heterocyclic (ipso-C)H atom followed by turnover limiting Ni slippage, and subsequently the allylated product is generated after alkene insertion and protonation. The strong electrostatic attraction between Ni and heterocyclic ipso-C in the Ni slippage pre-intermediate is critical for facilitating the Ni slippage. Once formed, the allylated product, assisted by NaOtBu, further evolves into a more stable alkenylated isomer. Employing a (tert-butyldimethylsilyl)-ether substituted allene as the substrate, the NaOtBu-induced chemoselectivity for dienylation vs. alkenylation was also probed and it was found that the O(tBu)–H⋯O(Si) hydrogen bonding interaction in the C–O(Si) cleavage pre-intermediate remarkably weakens the adjacent C–O(Si) σ-bond, thereby resulting in an exclusive C–O(Si) cleaved dienylation product. Further theoretical predictions suggest that the chemoselectivity might be reversed by replacing tBu in NaOtBu by the withdrawing C(CF3)3 group.
中文翻译:

阐明了可合成的Ni(0)催化的C–H烯丙基化,烯基化和二烯基化与丙二烯的多功能合成方法的理论解释
Ni(0)催化的苯并咪唑与1,1-二取代的丙二烯的偶联代表了实现可控的C–H烯丙基化,烯基化和二烯基化的新策略。为了了解可切换选择性的详细机理和起源,进行了密度泛函理论(DFT)计算。使用t Bu取代的亚丙基的结果表明,烯丙基化产物的形成涉及镍在无碱条件下通过配体-配体-氢转移(LLHT)进行Ni催化的CH活化机理。相反,在存在NaO t Bu的情况下,新提出了Ni / NaO t Bu共促进的C–H活化机制,这与以前报道的文献明显不同。新机制强调了NaOt Bu提取了Ni活化的杂环(ipso -C)H原子,然后限制了Ni的滑移,随后在烯烃插入和质子化之后生成了烯丙基化产物。Ni滑移预中间体中Ni与杂环ipso -C之间的强静电吸引力对于促进Ni滑移至关重要。形成后,在NaO t Bu的辅助下,烯丙基化产物进一步演变为更稳定的烯基化异构体。以(叔丁基二甲基甲硅烷基)-醚取代的烯丙基为底物,还探讨了NaO t Bu诱导的二烯基化与烯基化的化学选择性,发现O(t在中间中间体C–O(Si)裂解中,Bu)–H⋯O(Si)氢键相互作用显着削弱了相邻的C–O(Si)σ-键,从而导致独有的C–O(Si)裂解二烯化产物。进一步的理论预测表明,通过用C(CF 3)3基团取代NaO t Bu中的t Bu可以逆转化学选择性。
更新日期:2020-07-06
中文翻译:

阐明了可合成的Ni(0)催化的C–H烯丙基化,烯基化和二烯基化与丙二烯的多功能合成方法的理论解释
Ni(0)催化的苯并咪唑与1,1-二取代的丙二烯的偶联代表了实现可控的C–H烯丙基化,烯基化和二烯基化的新策略。为了了解可切换选择性的详细机理和起源,进行了密度泛函理论(DFT)计算。使用t Bu取代的亚丙基的结果表明,烯丙基化产物的形成涉及镍在无碱条件下通过配体-配体-氢转移(LLHT)进行Ni催化的CH活化机理。相反,在存在NaO t Bu的情况下,新提出了Ni / NaO t Bu共促进的C–H活化机制,这与以前报道的文献明显不同。新机制强调了NaOt Bu提取了Ni活化的杂环(ipso -C)H原子,然后限制了Ni的滑移,随后在烯烃插入和质子化之后生成了烯丙基化产物。Ni滑移预中间体中Ni与杂环ipso -C之间的强静电吸引力对于促进Ni滑移至关重要。形成后,在NaO t Bu的辅助下,烯丙基化产物进一步演变为更稳定的烯基化异构体。以(叔丁基二甲基甲硅烷基)-醚取代的烯丙基为底物,还探讨了NaO t Bu诱导的二烯基化与烯基化的化学选择性,发现O(t在中间中间体C–O(Si)裂解中,Bu)–H⋯O(Si)氢键相互作用显着削弱了相邻的C–O(Si)σ-键,从而导致独有的C–O(Si)裂解二烯化产物。进一步的理论预测表明,通过用C(CF 3)3基团取代NaO t Bu中的t Bu可以逆转化学选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号