当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Amyloid-β (Aβ42) Peptide Aggregation Rate and Mechanism on Surfaces with Widely Varied Properties: Insights from Brownian Dynamics Simulations.
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-06-11 , DOI: 10.1021/acs.jpcb.0c02926 Timothy Cholko 1 , Joseph Barnum 1 , Chia-En A Chang 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-06-11 , DOI: 10.1021/acs.jpcb.0c02926 Timothy Cholko 1 , Joseph Barnum 1 , Chia-En A Chang 1
Affiliation
|
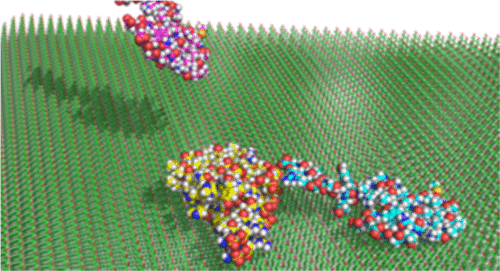
Amyloid-β (Aβ) plaques, which form by aggregation of harmless Aβ peptide monomers into larger fibrils, are characteristic of neurodegenerative disorders such as Alzheimer’s disease. Efforts to treat Alzheimer’s disease focus on stopping or reversing the aggregation process that leads to fibril formation. However, effective treatments are elusive due to certain unknown aspects of the process. Many hypotheses point to disruption of cell membranes by adsorbed Aβ monomers or oligomers, but how Aβ behaves and aggregates on surfaces of widely varying properties, such as those present in a cell, is unclear. Elucidating the effects of various surfaces on the dynamics of Aβ and the kinetics of the aggregation process from bulk solution to a surface-adsorbed multimer can help identify what drives aggregation, leading to new methods of intervention by inhibitory drugs or other means. In this work, we used all-atom Brownian dynamics simulations to study the association of two distinct Aβ42 monomer conformations with a surface-adsorbed or free-floating Aβ42 dimer. We calculated the association time, surface interaction energy, surface diffusion coefficient, surface residence time, and the mechanism of association on four different surfaces and two different bulk solution scenarios. In the presence of a surface, the majority of monomers underwent a two-dimensional surface-mediated association that depended primarily on an Aβ42 electrostatic interaction with the self-assembled monolayer (SAM) surfaces. Moreover, aggregation could be inhibited greatly by surfaces with high affinity for Aβ42 and heterogeneous charge distribution. Our results can be used to identify new opportunities for disrupting or reversing the Aβ42 aggregation process.
中文翻译:

具有广泛变化特性的表面上的淀粉样β(Aβ42)肽聚集速率和机理:来自布朗动力学模拟的见解。
淀粉样蛋白-β(Aβ)斑块是由无害的Aβ肽单体聚集成较大的原纤维形成的,是神经退行性疾病(例如阿尔茨海默氏病)的特征。治疗阿尔茨海默氏病的努力集中在停止或逆转导致原纤维形成的聚集过程。但是,由于该过程的某些未知方面,有效的治疗难以捉摸。许多假设都指出吸附的Aβ单体或低聚物会破坏细胞膜,但尚不清楚Aβ在行为广泛的表面(如细胞中存在的表面)上如何表现和聚集。阐明各种表面对Aβ动力学的影响以及从本体溶液到表面吸附的多聚体的聚集过程动力学,可以帮助您确定引起聚集的因素,导致通过抑制药物或其他手段进行干预的新方法。在这项工作中,我们使用全原子布朗动力学模拟研究了两个截然不同的Aβ42单体构象与表面吸附或自由漂浮的Aβ42二聚体的关联。我们计算了缔合时间,表面相互作用能,表面扩散系数,表面停留时间,以及在四个不同表面和两个不同本体解决方案上的缔合机理。在存在表面的情况下,大多数单体进行二维表面介导的缔合,该缔合主要取决于与自组装单层(SAM)表面的Aβ42静电相互作用。此外,对Aβ42具有高度亲和力的表面和异质电荷分布可极大地抑制聚集。
更新日期:2020-07-09
中文翻译:

具有广泛变化特性的表面上的淀粉样β(Aβ42)肽聚集速率和机理:来自布朗动力学模拟的见解。
淀粉样蛋白-β(Aβ)斑块是由无害的Aβ肽单体聚集成较大的原纤维形成的,是神经退行性疾病(例如阿尔茨海默氏病)的特征。治疗阿尔茨海默氏病的努力集中在停止或逆转导致原纤维形成的聚集过程。但是,由于该过程的某些未知方面,有效的治疗难以捉摸。许多假设都指出吸附的Aβ单体或低聚物会破坏细胞膜,但尚不清楚Aβ在行为广泛的表面(如细胞中存在的表面)上如何表现和聚集。阐明各种表面对Aβ动力学的影响以及从本体溶液到表面吸附的多聚体的聚集过程动力学,可以帮助您确定引起聚集的因素,导致通过抑制药物或其他手段进行干预的新方法。在这项工作中,我们使用全原子布朗动力学模拟研究了两个截然不同的Aβ42单体构象与表面吸附或自由漂浮的Aβ42二聚体的关联。我们计算了缔合时间,表面相互作用能,表面扩散系数,表面停留时间,以及在四个不同表面和两个不同本体解决方案上的缔合机理。在存在表面的情况下,大多数单体进行二维表面介导的缔合,该缔合主要取决于与自组装单层(SAM)表面的Aβ42静电相互作用。此外,对Aβ42具有高度亲和力的表面和异质电荷分布可极大地抑制聚集。











































 京公网安备 11010802027423号
京公网安备 11010802027423号