当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Decarboxylative Bromination of Sterically Hindered Carboxylic Acids with Hypervalent Iodine(III) Reagents
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-06-11 , DOI: 10.1021/acs.oprd.0c00130 Ayumi Watanabe 1 , Kenta Koyamada 1 , Kazunori Miyamoto 1 , Junichiro Kanazawa 1 , Masanobu Uchiyama 1, 2, 3
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-06-11 , DOI: 10.1021/acs.oprd.0c00130 Ayumi Watanabe 1 , Kenta Koyamada 1 , Kazunori Miyamoto 1 , Junichiro Kanazawa 1 , Masanobu Uchiyama 1, 2, 3
Affiliation
|
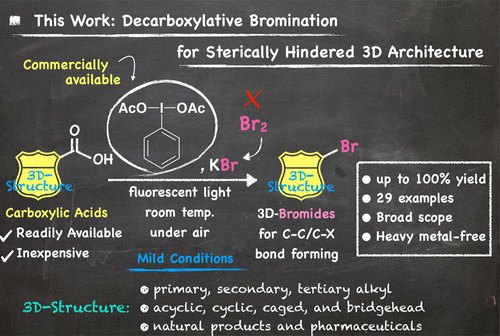
Sterically hindered three-dimensional (3D) alkyl halides are promising precursors for various reactions; however, they are difficult to synthesize via conventional reactions. We present an efficient and practical method for decarboxylative bromination of sterically hindered 3D aliphatic carboxylic acids using commercially available (diacetoxyiodo)benzene and potassium bromide, one of the most stable and cheapest bromine sources in nature. The present method features a metal-free/Br2-free system, mild reaction conditions, one-pot operation under air at room temperature, wide functional group compatibility, and gram-scale synthetic capability. This highly efficient reaction cleanly converts a broad range of carboxylic acids, the most inexpensive and readily available sources of highly strained/naturally occurring/drug-related scaffolds, into the corresponding alkyl bromides in good to high yields.
中文翻译:

高价碘试剂对立体受阻羧酸的脱羧溴化反应
立体受阻的三维(3D)烷基卤化物是各种反应的有希望的前体;但是,它们难以通过常规反应合成。我们提供了使用市售的(diacetoxyiodo)苯和溴化钾(自然界中最稳定和最便宜的溴源之一)对位阻3D脂肪族羧酸进行脱羧溴化的高效实用方法。本方法的特点是不含金属/ Br 2无体系,温和的反应条件,在室温下空气中一锅操作,广泛的官能团相容性和克级合成能力。这种高效的反应可以以高收率或高收率干净地将各种羧酸(高度紧张/天然存在/与药物相关的支架的最便宜和最容易获得的来源)转化为相应的烷基溴化物。
更新日期:2020-07-17
中文翻译:

高价碘试剂对立体受阻羧酸的脱羧溴化反应
立体受阻的三维(3D)烷基卤化物是各种反应的有希望的前体;但是,它们难以通过常规反应合成。我们提供了使用市售的(diacetoxyiodo)苯和溴化钾(自然界中最稳定和最便宜的溴源之一)对位阻3D脂肪族羧酸进行脱羧溴化的高效实用方法。本方法的特点是不含金属/ Br 2无体系,温和的反应条件,在室温下空气中一锅操作,广泛的官能团相容性和克级合成能力。这种高效的反应可以以高收率或高收率干净地将各种羧酸(高度紧张/天然存在/与药物相关的支架的最便宜和最容易获得的来源)转化为相应的烷基溴化物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号