当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Superoxide-Based K–O2 Batteries: Highly Reversible Oxygen Redox Solves Challenges in Air Electrodes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-06-10 , DOI: 10.1021/jacs.0c05141 Lei Qin 1 , Luke Schkeryantz 1 , Jingfeng Zheng 1 , Neng Xiao 1 , Yiying Wu 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-06-10 , DOI: 10.1021/jacs.0c05141 Lei Qin 1 , Luke Schkeryantz 1 , Jingfeng Zheng 1 , Neng Xiao 1 , Yiying Wu 1
Affiliation
|
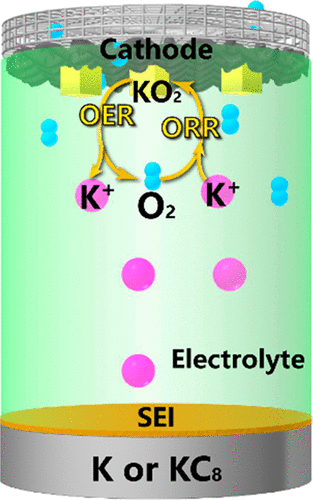
In the last twenty years, research in metal-O2 batteries has been one of the most exciting interdisciplinary fields of electrochemistry, energy storage, materials chemistry, and surface science. The mechanisms of oxygen reduction and evolution play a key role in understanding and controlling these batteries. With intensive efforts from many prominent research groups, it becomes clear that the instability of superoxide in the presence of Li ions (Li+) and Na ions (Na+) is the fundamental root cause for the poor stability, reversibility, and energy efficiency in aprotic Li-O2 and Na-O2 batteries. Stabilizing superoxide with large K ions (K+) provides a simple but elegant solution. Superoxide-based K-O2 batteries, invented in 2013, adopt the one-electron redox process of O2/potassium superoxide (KO2). Despite being the youngest metal-O2 technology, K-O2 is the most promising rechargeable metal-air battery with the combined advantages in low costs, high energy efficiencies, abundant elements, and good energy densities. However, the development of the K-O2 battery has been overshadowed by Li-O2 and Na-O2 batteries because one might think K-O2 is just an analogous extension. Moreover, due to the lower specific energy and the high reactivity of K metal, K-O2 is often underestimated and deemed unsuitable for practical applications. The objective of this Perspective is to highlight the unique advantages of K-O2 chemistry and to clarify the misconceptions prompted by the name "superoxide" and the judgement bias based on the claimed theoretical specific energies. We will also discuss the current challenges and our perspectives on how to overcome them.
中文翻译:

基于超氧化物的 K-O2 电池:高度可逆的氧氧化还原解决了空气电极中的挑战
在过去的二十年里,金属-O2 电池的研究一直是电化学、储能、材料化学和表面科学最令人兴奋的跨学科领域之一。氧还原和析出的机制在理解和控制这些电池方面起着关键作用。随着许多著名研究小组的深入努力,很明显,在锂离子 (Li+) 和钠离子 (Na+) 存在下超氧化物的不稳定性是非质子锂稳定性、可逆性和能量效率差的根本原因-O2 和 Na-O2 电池。用大 K 离子 (K+) 稳定超氧化物提供了一种简单而优雅的解决方案。2013 年发明的超氧化物 K-O2 电池采用 O2/超氧化钾 (KO2) 的单电子氧化还原过程。尽管是最年轻的金属-O2 技术,K-O2具有成本低、能效高、元素丰富、能量密度好等优点,是最具发展前景的可充电金属-空气电池。然而,K-O2 电池的发展已经被 Li-O2 和 Na-O2 电池所掩盖,因为人们可能认为 K-O2 只是一种类似的延伸。此外,由于 K 金属的较低比能和高反应性,K-O2 经常被低估并被认为不适合实际应用。本透视图的目的是突出 K-O2 化学的独特优势,并澄清由“超氧化物”名称引起的误解以及基于所声称的理论比能的判断偏差。我们还将讨论当前的挑战以及我们对如何克服这些挑战的看法。
更新日期:2020-06-10
中文翻译:

基于超氧化物的 K-O2 电池:高度可逆的氧氧化还原解决了空气电极中的挑战
在过去的二十年里,金属-O2 电池的研究一直是电化学、储能、材料化学和表面科学最令人兴奋的跨学科领域之一。氧还原和析出的机制在理解和控制这些电池方面起着关键作用。随着许多著名研究小组的深入努力,很明显,在锂离子 (Li+) 和钠离子 (Na+) 存在下超氧化物的不稳定性是非质子锂稳定性、可逆性和能量效率差的根本原因-O2 和 Na-O2 电池。用大 K 离子 (K+) 稳定超氧化物提供了一种简单而优雅的解决方案。2013 年发明的超氧化物 K-O2 电池采用 O2/超氧化钾 (KO2) 的单电子氧化还原过程。尽管是最年轻的金属-O2 技术,K-O2具有成本低、能效高、元素丰富、能量密度好等优点,是最具发展前景的可充电金属-空气电池。然而,K-O2 电池的发展已经被 Li-O2 和 Na-O2 电池所掩盖,因为人们可能认为 K-O2 只是一种类似的延伸。此外,由于 K 金属的较低比能和高反应性,K-O2 经常被低估并被认为不适合实际应用。本透视图的目的是突出 K-O2 化学的独特优势,并澄清由“超氧化物”名称引起的误解以及基于所声称的理论比能的判断偏差。我们还将讨论当前的挑战以及我们对如何克服这些挑战的看法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号