当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Anionic Redox Reactions in Manganese-Based Binary Layered Oxides for Advanced Sodium-Ion Batteries
Chemistry of Materials ( IF 7.2 ) Pub Date : 2020-06-11 , DOI: 10.1021/acs.chemmater.0c00415 Duho Kim 1 , Jangmin Lee 1
Chemistry of Materials ( IF 7.2 ) Pub Date : 2020-06-11 , DOI: 10.1021/acs.chemmater.0c00415 Duho Kim 1 , Jangmin Lee 1
Affiliation
|
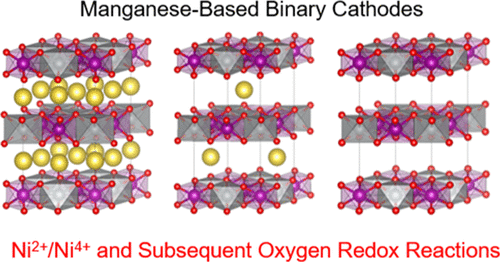
Oxygen 2p-electron, unhybridized with transition metals (TMs), is a critical species for the generation of an anion-based redox reaction of O2–/O– for high-energy-density cathodes in lithium-ion and sodium-ion batteries (LIBs and SIBs, respectively). More importantly, oxygen redox activity has been highlighted as a breakthrough to increase the intrinsic low redox potential for SIBs because its reaction theoretically and experimentally occurs at ≈4.2 V versus Na+/Na. Here, we present in detail the thermodynamic, structural, and chemical origins of stabilized Ni2+ (redoxable) and Mn4+ (nonredoxable), determined by the different electronegativity values in Mn-based binary layered oxides, without excess monovalent element in the TM layer, for the rational use of an anion-based redox reaction. The Ni solubility into Na[Mn1–xNix]O2 has the highest value at x = 0.5 owing to the decrease in Mn3+ instability with the maximally stabilized Ni2+, which is understood by the thermodynamic mixing enthalpy value, M–O (M: Mn and Ni) and Na–O bonding lengths, and qualitative and quantitative electronic structure investigations. Utilizing the cumulative redox reaction, combined with the cationic and anionic species, the thermodynamically stabilized Na[Mn0.5Ni0.5]O2 is predicted to show a double redox reaction of Ni2+/Ni4+ and a subsequent anion-based redox reaction of O2–/On– combined with a partial Ni redox contribution, indicating a much higher redox potential behavior as compared with that of a single redox reaction of Mn3+/Mn4+ in NaMnO2. Our more concrete understanding of the thermodynamic, structural, and chemical origins, coupled with the stabilized Ni2+ (variant) and Mn4+ (invariant) species for redox reactions in the energetics in crystal field theory, is a critical factor in boosting the use of the Mn-based layered oxides and overcoming the limitations of low energy density for SIBs.
中文翻译:

用于高级钠离子电池的锰基二元氧化物中的阴离子氧化还原反应
氧的2p电子,与过渡金属(TMS)未杂交,是用于将基于阴离子氧化还原反应的产生的关键物种ö 2- / O -用于在锂离子和钠离子电池的高能量密度的阴极(分别为LIB和SIB)。更重要的是,氧气氧化还原活性已被认为是增加SIBs固有的低氧化还原电位的突破,因为它的反应在理论上和实验上相对于Na + / Na约为4.2V 。在这里,我们详细介绍了稳定的Ni 2+(可氧化)和Mn 4+的热力学,结构和化学起源。(不可氧化还原),是由Mn基二元层状氧化物中不同的电负性值确定的,TM层中没有过量的一价元素,用于合理使用基于阴离子的氧化还原反应。Ni在Na [Mn 1– x Ni x ] O 2中的溶解度在x = 0.5时具有最高值,这是因为随着最大稳定的Ni 2 +,Mn 3+不稳定性降低,这可以通过热力学混合焓值来理解, M–O(M:Mn和Ni)和Na–O键长,以及定性和定量电子结构研究。利用累积的氧化还原反应,结合阳离子和阴离子物质,热力学稳定的Na [Mn0.5镍0.5 ] O 2被预测为显示Ni的双氧化还原反应2+ /镍4+和随后的基于阴离子氧化还原反应ö 2- / O ñ -结合的部分的Ni氧化还原贡献,表明高得多的与NaMnO 2中Mn 3+ / Mn 4+的单个氧化还原反应相比,氧化还原电位行为。我们对热力学,结构和化学起源的更具体的理解,再加上稳定的Ni 2+(变体)和Mn 4+ 晶体场论中高能学中氧化还原反应的(不变)物种是促进使用Mn基层状氧化物并克服SIB的低能量密度限制的关键因素。
更新日期:2020-07-14
中文翻译:

用于高级钠离子电池的锰基二元氧化物中的阴离子氧化还原反应
氧的2p电子,与过渡金属(TMS)未杂交,是用于将基于阴离子氧化还原反应的产生的关键物种ö 2- / O -用于在锂离子和钠离子电池的高能量密度的阴极(分别为LIB和SIB)。更重要的是,氧气氧化还原活性已被认为是增加SIBs固有的低氧化还原电位的突破,因为它的反应在理论上和实验上相对于Na + / Na约为4.2V 。在这里,我们详细介绍了稳定的Ni 2+(可氧化)和Mn 4+的热力学,结构和化学起源。(不可氧化还原),是由Mn基二元层状氧化物中不同的电负性值确定的,TM层中没有过量的一价元素,用于合理使用基于阴离子的氧化还原反应。Ni在Na [Mn 1– x Ni x ] O 2中的溶解度在x = 0.5时具有最高值,这是因为随着最大稳定的Ni 2 +,Mn 3+不稳定性降低,这可以通过热力学混合焓值来理解, M–O(M:Mn和Ni)和Na–O键长,以及定性和定量电子结构研究。利用累积的氧化还原反应,结合阳离子和阴离子物质,热力学稳定的Na [Mn0.5镍0.5 ] O 2被预测为显示Ni的双氧化还原反应2+ /镍4+和随后的基于阴离子氧化还原反应ö 2- / O ñ -结合的部分的Ni氧化还原贡献,表明高得多的与NaMnO 2中Mn 3+ / Mn 4+的单个氧化还原反应相比,氧化还原电位行为。我们对热力学,结构和化学起源的更具体的理解,再加上稳定的Ni 2+(变体)和Mn 4+ 晶体场论中高能学中氧化还原反应的(不变)物种是促进使用Mn基层状氧化物并克服SIB的低能量密度限制的关键因素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号