当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New Perspectives on Drug-Induced Liver Injury Risk Assessment of Acyl Glucuronides.
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2020-06-11 , DOI: 10.1021/acs.chemrestox.0c00131 Markus Walles 1 , Alan P Brown 2 , Alfred Zimmerlin 1 , Peter End 1
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2020-06-11 , DOI: 10.1021/acs.chemrestox.0c00131 Markus Walles 1 , Alan P Brown 2 , Alfred Zimmerlin 1 , Peter End 1
Affiliation
|
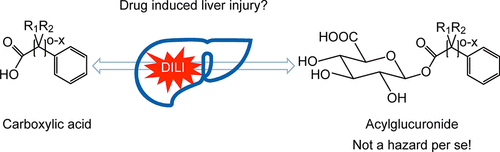
Drug-induced liver injury (DILI) remains one of the key challenges in drug development due to the mechanisms of action being multifactorial in nature. This is particularly the case for idiosyncratic DILI which occurs in a very low frequency in humans (e.g., 1:10,000). Despite perceptions that acyl glucuronide metabolites are defacto risks for DILI, scientific evidence suggests that acyl glucuronide formation alone does not pose an increased risk compared to other drug metabolites. This applies in particular to those acyl glucuronides which are not reactive and do not form covalent adducts with proteins. The goal of this paper is to provide guidance on preclinical and clinical strategies to evaluate the potential for acyl glucuronide formation to contribute to DILI. A key element of our proposed safety assessment is to investigate whether a particular acyl glucuronide is reactive or not and whether systemic exposure in humans can be demonstrated in animal toxicology studies following administration of the parent drug. While standard animal toxicology studies can identify overtly hepatotoxic compounds, these studies are not predictive for drugs that produce idiosyncratic forms of DILI. In addition, we do not recommend conducting toxicology studies of administered individual acyl glucuronides due to differences in pharmacokinetic and dispositional properties from the endogenously produced metabolites. Once a drug candidate has entered clinical trials, the focus should be on clinical safety data and emerging risk–benefit analysis.
中文翻译:

药物引起的酰基葡萄糖醛酸苷肝损伤风险评估的新观点。
由于作用机理本质上是多因素的,因此药物诱发的肝损伤(DILI)仍然是药物开发中的关键挑战之一。特异DILI的情况尤其如此,它在人类中的发生频率非常低(例如1:10,000)。尽管人们认为酰基葡萄糖醛酸化物代谢物是DILI的事实上风险,但科学证据表明,与其他药物代谢物相比,单独形成酰基葡萄糖醛酸化物不会带来增加的风险。这尤其适用于那些不反应并且不与蛋白质形成共价加合物的酰基葡糖醛酸苷。本文的目的是为临床前和临床策略提供指导,以评估酰基葡糖醛酸苷形成对DILI的贡献。我们提出的安全性评估的关键要素是调查特定的酰基葡萄糖醛酸苷是否具有反应性,以及在母体药物给药后的动物毒理学研究中是否可以证明人体的全身暴露。虽然标准的动物毒理学研究可以鉴定出明显的肝毒性化合物,但这些研究对于产生特异形式DILI的药物没有预测作用。另外,由于内源性代谢物的药代动力学和处置特性不同,我们不建议对所施用的各个酰基葡萄糖醛酸内酯进行毒理学研究。候选药物进入临床试验后,重点应放在临床安全性数据和新兴的风险效益分析上。
更新日期:2020-07-20
中文翻译:

药物引起的酰基葡萄糖醛酸苷肝损伤风险评估的新观点。
由于作用机理本质上是多因素的,因此药物诱发的肝损伤(DILI)仍然是药物开发中的关键挑战之一。特异DILI的情况尤其如此,它在人类中的发生频率非常低(例如1:10,000)。尽管人们认为酰基葡萄糖醛酸化物代谢物是DILI的事实上风险,但科学证据表明,与其他药物代谢物相比,单独形成酰基葡萄糖醛酸化物不会带来增加的风险。这尤其适用于那些不反应并且不与蛋白质形成共价加合物的酰基葡糖醛酸苷。本文的目的是为临床前和临床策略提供指导,以评估酰基葡糖醛酸苷形成对DILI的贡献。我们提出的安全性评估的关键要素是调查特定的酰基葡萄糖醛酸苷是否具有反应性,以及在母体药物给药后的动物毒理学研究中是否可以证明人体的全身暴露。虽然标准的动物毒理学研究可以鉴定出明显的肝毒性化合物,但这些研究对于产生特异形式DILI的药物没有预测作用。另外,由于内源性代谢物的药代动力学和处置特性不同,我们不建议对所施用的各个酰基葡萄糖醛酸内酯进行毒理学研究。候选药物进入临床试验后,重点应放在临床安全性数据和新兴的风险效益分析上。











































 京公网安备 11010802027423号
京公网安备 11010802027423号