当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Circular Dichroism Spectroscopy Identifies the β-Adrenoceptor Agonist Salbutamol As a Direct Inhibitor of Tau Filament Formation in Vitro.
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2020-06-10 , DOI: 10.1021/acschemneuro.0c00154 David J Townsend 1 , Barbora Mala 1 , Eleri Hughes 1 , Rohanah Hussain 2 , Giuliano Siligardi 2 , Nigel J Fullwood 3 , David A Middleton 1
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2020-06-10 , DOI: 10.1021/acschemneuro.0c00154 David J Townsend 1 , Barbora Mala 1 , Eleri Hughes 1 , Rohanah Hussain 2 , Giuliano Siligardi 2 , Nigel J Fullwood 3 , David A Middleton 1
Affiliation
|
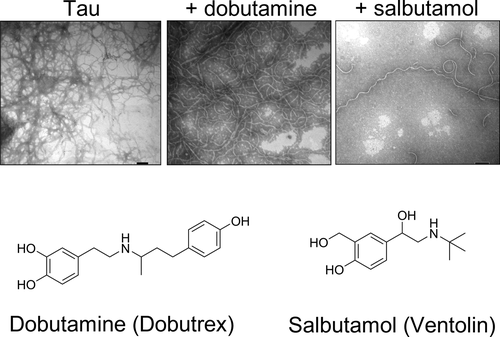
Potential drug treatments for Alzheimer’s disease (AD) may be found by identifying compounds that block the assembly of the microtubule-associated protein tau into neurofibrillar tangles associated with neuron destabilization and cell death. Here, a small library of structurally diverse compounds was screened in vitro for the ability to inhibit tau aggregation, using high-throughput synchrotron radiation circular dichroism as a novel tool to monitor the structural changes in the protein as it assembles into filaments. The catecholamine epinephrine was found to be the most effective tau aggregation inhibitor of all 88 screened compounds. Subsequently, we tested chemically similar phenolamine drugs from the β-adrenergic receptor agonist class, using conventional circular dichroism spectroscopy, thioflavin T fluorescence, and transmission electron microscopy. Two compounds, salbutamol and dobutamine, used widely in the treatment of respiratory and cardiovascular disease, impede the aggregation of tau in vitro. Dobutamine reduces both the rate and yield of tau filament formation over 24 h; however, it has little effect on the structural transition of tau into β-sheet structures over 24 h. Salbutamol also reduces the yield and rate of filament formation and additionally inhibits tau’s structural change into β-sheet-rich aggregates. Salbutamol has a good safety profile and a half-life that facilitates permeation through the blood–brain barrier and could represent an expediated approach to developing AD therapeutics. These results provide the motivation for the in vivo evaluation of pre-existing β-adrenergic receptor agonists as a potential therapy for AD through the reduction of tau deposition.
中文翻译:

圆二色光谱确定β-肾上腺素能受体激动剂沙丁胺醇是Tau丝形成的直接抑制剂。
通过鉴定能阻止微管相关蛋白tau组装成与神经元不稳定和细胞死亡相关的神经原纤维缠结的化合物,可以发现阿尔茨海默氏病(AD)的潜在药物治疗方法。在这里,体外筛选了一个结构多样的化合物的小型文库为了抑制tau聚集,使用高通量同步加速器辐射圆二色性作为一种新型工具来监测蛋白质组装成细丝时的结构变化。发现儿茶酚胺肾上腺素是所有88种筛选化合物中最有效的tau聚集抑制剂。随后,我们使用常规的圆二色光谱,硫黄素T荧光和透射电子显微镜测试了β-肾上腺素能受体激动剂类别的化学相似的酚胺药物。沙丁胺醇和多巴酚丁胺这两种化合物广泛用于治疗呼吸道和心血管疾病,阻碍tau的体外聚集。多巴酚丁胺可降低24小时内tau细丝形成的速度和产量;然而,在24小时内,它对tau转变为β-折叠结构的影响很小。沙丁胺醇还降低了长丝形成的产率和速率,并另外抑制了tau的结构变化为富含β-折叠的聚集体。沙丁胺醇具有良好的安全性和半衰期,可促进血脑屏障的渗透,并可能是开发AD治疗药物的一种快速方法。这些结果为体内评估先前存在的β-肾上腺素能受体激动剂提供了动机,作为通过减少tau沉积来治疗AD的潜在疗法。
更新日期:2020-07-15
中文翻译:

圆二色光谱确定β-肾上腺素能受体激动剂沙丁胺醇是Tau丝形成的直接抑制剂。
通过鉴定能阻止微管相关蛋白tau组装成与神经元不稳定和细胞死亡相关的神经原纤维缠结的化合物,可以发现阿尔茨海默氏病(AD)的潜在药物治疗方法。在这里,体外筛选了一个结构多样的化合物的小型文库为了抑制tau聚集,使用高通量同步加速器辐射圆二色性作为一种新型工具来监测蛋白质组装成细丝时的结构变化。发现儿茶酚胺肾上腺素是所有88种筛选化合物中最有效的tau聚集抑制剂。随后,我们使用常规的圆二色光谱,硫黄素T荧光和透射电子显微镜测试了β-肾上腺素能受体激动剂类别的化学相似的酚胺药物。沙丁胺醇和多巴酚丁胺这两种化合物广泛用于治疗呼吸道和心血管疾病,阻碍tau的体外聚集。多巴酚丁胺可降低24小时内tau细丝形成的速度和产量;然而,在24小时内,它对tau转变为β-折叠结构的影响很小。沙丁胺醇还降低了长丝形成的产率和速率,并另外抑制了tau的结构变化为富含β-折叠的聚集体。沙丁胺醇具有良好的安全性和半衰期,可促进血脑屏障的渗透,并可能是开发AD治疗药物的一种快速方法。这些结果为体内评估先前存在的β-肾上腺素能受体激动剂提供了动机,作为通过减少tau沉积来治疗AD的潜在疗法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号