当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identify Zr Promotion Effects in Atomic Scale for Co-Based Catalysts in Fischer–Tropsch Synthesis
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-06-10 , DOI: 10.1021/acscatal.0c01874 Yuang Piao 1, 2 , Qian Jiang 2 , Hao Li 3 , Hiroaki Matsumoto 4 , Jinsheng Liang 1 , Wei Liu 2 , Cuong Pham-Huu 5 , Yuefeng Liu 2 , Fei Wang 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-06-10 , DOI: 10.1021/acscatal.0c01874 Yuang Piao 1, 2 , Qian Jiang 2 , Hao Li 3 , Hiroaki Matsumoto 4 , Jinsheng Liang 1 , Wei Liu 2 , Cuong Pham-Huu 5 , Yuefeng Liu 2 , Fei Wang 1
Affiliation
|
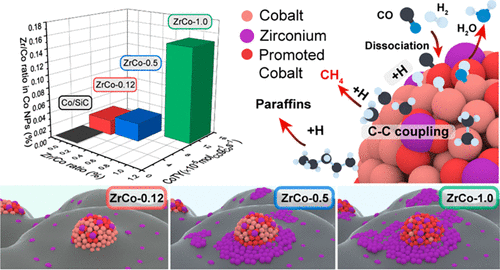
Introducing promoters on cobalt-based catalysts for Fischer–Tropsch synthesis (FTS) have been found efficient for adjusting their performance in converting syngas into long-chain hydrocarbons. Details of the promotion mechanism established on atomic precise identification upon the active sites structure as well as the electronic status is seldomly reported yet. In the present work, we report the direct identification of valence status and coordination configuration of ZrO2 promoter additive over the cobalt-based FTS catalysts under a wide range of Zr/Co molar ratios from 0.12 to 1.5. Evidences from multiple technologies, including in situ/ex situ atomic resolution STEM imaging, EDS elemental mapping, electron energy loss spectroscopy (EELS), as well as physicochemical analyses (in situ XRD, CO chemisorption, CO temperature-programmed surface reaction, etc.) disclose that the ZrO2 promoter presents as single-site dispersion on the surface of Co nanoparticles (NPs) and the SiC support at low content, plays as the real active site to promote CO dissociation at the Co-ZrO2 interface. While at high ZrO2 content (Zr/Co molar ratio of 1.0), Zr species on the SiC support turns to nucleate to form an amorphous coating, whereas those on the Co NPs surface maintain monodispersion. When further increasing the ZrO2 content (Zr/Co molar ratio up to 1.5), cobalt NPs start to be encapsulated by ZrO2 coating, leading to the decline of FTS activity. The in situ EELS analysis and density functional theory calculations disclose that the Zr atom tends to bind at the Co NPs surface rather than embed into the lattice meanwhile a charge transfer from Zr species to Co NPs occurs, which facilitates the stronger interaction between Zr and cobalt and thus enhances the adsorption with the H2 molecule as well as the CO dissociation. It thus offers enhanced catalytic activity and long-chain hydrocarbon selectivity during the FTS reaction.
中文翻译:

确定费-托合成中钴基催化剂在原子尺度上的Zr促进作用
已发现在费托合成(FTS)的钴基催化剂上引入促进剂可有效调节其将合成气转化为长链烃的性能。很少报道基于活性部位结构上的原子精确识别建立的促进机制以及电子状态的细节。在当前的工作中,我们报告ZrO 2的价态直接识别和配位构型Zr / Co摩尔比范围从0.12到1.5的钴基FTS催化剂上添加助催化剂添加剂。来自多种技术的证据,包括原位/异位原子分辨率STEM成像,EDS元素映射,电子能量损失谱(EELS)以及理化分析(原位XRD,CO化学吸附,CO温度程序化的表面反应等)。 )公开了ZrO 2启动子以单中心分散体的形式存在于Co纳米颗粒(NPs)和SiC载体上,含量低,充当了促进Co-ZrO 2界面上CO离解的真正活性中心。ZrO 2高时含量(Zr / Co摩尔比为1.0)时,SiC载体上的Zr物种成核形成无定形涂层,而Co NPs表面的Zr物种保持单分散。当进一步增加ZrO 2含量(Zr / Co摩尔比最高为1.5)时,钴NPs开始被ZrO 2涂层包裹,导致FTS活性下降。原位EELS分析和密度泛函理论计算表明,Zr原子倾向于结合在Co NPs表面而不是嵌入晶格中,同时发生从Zr物种到Co NPs的电荷转移,这促进了Zr和钴之间更强的相互作用从而增强了对H 2的吸附分子以及一氧化碳的离解。因此,它在FTS反应期间提供增强的催化活性和长链烃选择性。
更新日期:2020-07-17
中文翻译:

确定费-托合成中钴基催化剂在原子尺度上的Zr促进作用
已发现在费托合成(FTS)的钴基催化剂上引入促进剂可有效调节其将合成气转化为长链烃的性能。很少报道基于活性部位结构上的原子精确识别建立的促进机制以及电子状态的细节。在当前的工作中,我们报告ZrO 2的价态直接识别和配位构型Zr / Co摩尔比范围从0.12到1.5的钴基FTS催化剂上添加助催化剂添加剂。来自多种技术的证据,包括原位/异位原子分辨率STEM成像,EDS元素映射,电子能量损失谱(EELS)以及理化分析(原位XRD,CO化学吸附,CO温度程序化的表面反应等)。 )公开了ZrO 2启动子以单中心分散体的形式存在于Co纳米颗粒(NPs)和SiC载体上,含量低,充当了促进Co-ZrO 2界面上CO离解的真正活性中心。ZrO 2高时含量(Zr / Co摩尔比为1.0)时,SiC载体上的Zr物种成核形成无定形涂层,而Co NPs表面的Zr物种保持单分散。当进一步增加ZrO 2含量(Zr / Co摩尔比最高为1.5)时,钴NPs开始被ZrO 2涂层包裹,导致FTS活性下降。原位EELS分析和密度泛函理论计算表明,Zr原子倾向于结合在Co NPs表面而不是嵌入晶格中,同时发生从Zr物种到Co NPs的电荷转移,这促进了Zr和钴之间更强的相互作用从而增强了对H 2的吸附分子以及一氧化碳的离解。因此,它在FTS反应期间提供增强的催化活性和长链烃选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号