当前位置:
X-MOL 学术
›
Microb. Biotechnol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Revisiting the host adhesion determinants of Streptococcus thermophilus siphophages.
Microbial Biotechnology ( IF 4.8 ) Pub Date : 2020-06-11 , DOI: 10.1111/1751-7915.13593 Katherine Lavelle 1 , Adeline Goulet 2, 3 , Brian McDonnell 1 , Silvia Spinelli 2, 3 , Douwe van Sinderen 1, 4 , Jennifer Mahony 1, 4 , Christian Cambillau 1, 2, 3
Microbial Biotechnology ( IF 4.8 ) Pub Date : 2020-06-11 , DOI: 10.1111/1751-7915.13593 Katherine Lavelle 1 , Adeline Goulet 2, 3 , Brian McDonnell 1 , Silvia Spinelli 2, 3 , Douwe van Sinderen 1, 4 , Jennifer Mahony 1, 4 , Christian Cambillau 1, 2, 3
Affiliation
|
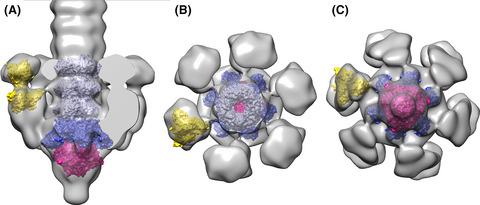
Available 3D structures of bacteriophage modules combined with predictive bioinformatic algorithms enabled the identification of adhesion modules in 57 siphophages infecting Streptococcus thermophilus (St). We identified several carbohydrate‐binding modules (CBMs) in so‐called evolved distal tail (Dit) and tail‐associated lysozyme (Tal) proteins of St phage baseplates. We examined the open reading frame (ORF) downstream of the Tal‐encoding ORF and uncovered the presence of a putative p2‐like receptor‐binding protein (RBP). A 21 Å resolution electron microscopy structure of the baseplate of cos‐phage STP1 revealed the presence of six elongated electron densities, surrounding the core of the baseplate, that harbour the p2‐like RBPs at their tip. To verify the functionality of these modules, we expressed GFP‐ or mCherry‐coupled Tal and putative RBP CBMs and observed by fluorescence microscopy that both modules bind to their corresponding St host, the putative RBP CBM with higher affinity than the Tal‐associated one. The large number of CBM functional domains in St phages suggests that they play a contributory role in the infection process, a feature that we previously described in lactococcal phages and beyond, possibly representing a universal feature of the siphophage host‐recognition apparatus.
中文翻译:

重新审视嗜热链球菌噬菌体的宿主粘附决定因素。
噬菌体模块的可用 3D 结构与预测生物信息学算法相结合,能够识别感染嗜热链球菌(St) 的 57 个噬菌体中的粘附模块。我们在 St 噬菌体底板的所谓进化远端尾部 (Dit) 和尾部相关溶菌酶 (Tal) 蛋白中鉴定了几个碳水化合物结合模块 (CBM)。我们检查了 Tal 编码 ORF 下游的开放阅读框 (ORF),发现了假定的 p2 样受体结合蛋白 (RBP) 的存在。 cos噬菌体 STP1 底板的 21 Å 分辨率电子显微镜结构揭示了围绕底板核心的六个细长电子密度的存在,这些电子密度在其尖端包含 p2 样 RBP。为了验证这些模块的功能,我们表达了 GFP 或 mCherry 偶联的 Tal 和假定的 RBP CBM,并通过荧光显微镜观察到这两个模块与其相应的 St 宿主结合,即假定的 RBP CBM 比 Tal 相关的具有更高的亲和力。 St 噬菌体中大量的 CBM 功能域表明它们在感染过程中发挥着重要作用,这一特征我们之前在乳球菌噬菌体及其他噬菌体中描述过,可能代表了噬菌体宿主识别装置的普遍特征。
更新日期:2020-06-11
中文翻译:

重新审视嗜热链球菌噬菌体的宿主粘附决定因素。
噬菌体模块的可用 3D 结构与预测生物信息学算法相结合,能够识别感染嗜热链球菌(St) 的 57 个噬菌体中的粘附模块。我们在 St 噬菌体底板的所谓进化远端尾部 (Dit) 和尾部相关溶菌酶 (Tal) 蛋白中鉴定了几个碳水化合物结合模块 (CBM)。我们检查了 Tal 编码 ORF 下游的开放阅读框 (ORF),发现了假定的 p2 样受体结合蛋白 (RBP) 的存在。 cos噬菌体 STP1 底板的 21 Å 分辨率电子显微镜结构揭示了围绕底板核心的六个细长电子密度的存在,这些电子密度在其尖端包含 p2 样 RBP。为了验证这些模块的功能,我们表达了 GFP 或 mCherry 偶联的 Tal 和假定的 RBP CBM,并通过荧光显微镜观察到这两个模块与其相应的 St 宿主结合,即假定的 RBP CBM 比 Tal 相关的具有更高的亲和力。 St 噬菌体中大量的 CBM 功能域表明它们在感染过程中发挥着重要作用,这一特征我们之前在乳球菌噬菌体及其他噬菌体中描述过,可能代表了噬菌体宿主识别装置的普遍特征。











































 京公网安备 11010802027423号
京公网安备 11010802027423号