Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2020-06-11 , DOI: 10.1016/j.jhazmat.2020.123083 Su-Jin Min 1 , Jong-Gook Kim 1 , Kitae Baek 1
|
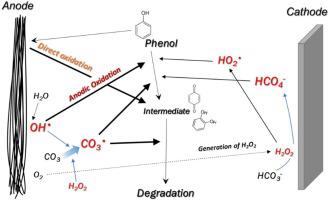
In-situ chemical oxidation (ISCO) requires an injection of oxidants into a contaminated site. However, the oxidants decompose and react with contaminants during transport to the contaminated region, which causes oxidant over-consumption. In-situ oxidant generation can solve this problem, and electrochemical methods can be applied to achieve this. Electrochemical oxidation is highly dependent on electrode material type. In this study, we evaluated graphite and carbon fiber as candidates for electrochemical oxidant generation and phenol as the model compound. The carbon fiber anode oxidized the phenol more effectively than graphite, with removal proportional to the applied current. Carbonate electrolytes were more effective at oxidizing phenols than sulfate electrolytes. The faster carbon fiber anode phenol oxidation is due to its large surface area. Carbonate radicals in the carbonate electrolyte contribute to phenol oxidation as well as further intermediate oxidation. The carbon fiber cathode was not an effective phenol oxidizer even though it generated more hydrogen peroxide. This is because there was no catalyst to transform the hydrogen peroxide into hydroxyl radicals. Results indicate that electrochemical oxidation using carbon fiber is an effective method for treating phenol found in groundwater with high concentrations of (bi)carbonate.
中文翻译:

碳纤维电极和碳酸盐电解质在电化学苯酚氧化中的作用。
原位化学氧化(ISCO)需要将氧化剂注入污染区域。但是,氧化剂在运输到污染区域的过程中会分解并与污染物反应,这会导致氧化剂过度消耗。原位氧化剂的产生可以解决这个问题,可以采用电化学方法来解决这个问题。电化学氧化高度取决于电极材料的类型。在这项研究中,我们评估了石墨和碳纤维作为电化学氧化剂生成的候选物,而苯酚作为模型化合物。碳纤维阳极比石墨更有效地氧化了苯酚,去除率与施加的电流成比例。碳酸盐电解质在氧化苯酚方面比硫酸盐电解质更有效。碳纤维阳极苯酚氧化较快是由于其表面积大。碳酸盐电解质中的碳酸盐自由基有助于苯酚氧化以及进一步的中间氧化。尽管碳纤维阴极产生更多的过氧化氢,但它并不是有效的酚类氧化剂。这是因为没有催化剂将过氧化氢转化为羟基自由基。结果表明,使用碳纤维进行电化学氧化是一种有效的方法,用于处理地下水中高浓度的碳酸氢根中的苯酚。











































 京公网安备 11010802027423号
京公网安备 11010802027423号