当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Straight access to highly fluorescent angular indolocarbazoles via merging Au- and Mo-catalysis
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-06-10 , DOI: 10.1039/d0qo00405g Fernando Martínez-Lara 1, 2, 3, 4, 5 , Anisley Suárez 1, 2, 3, 4, 5 , Samuel Suárez-Pantiga 1, 2, 3, 4, 5 , M. José Tapia 1, 2, 3, 4, 5 , Roberto Sanz 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-06-10 , DOI: 10.1039/d0qo00405g Fernando Martínez-Lara 1, 2, 3, 4, 5 , Anisley Suárez 1, 2, 3, 4, 5 , Samuel Suárez-Pantiga 1, 2, 3, 4, 5 , M. José Tapia 1, 2, 3, 4, 5 , Roberto Sanz 1, 2, 3, 4, 5
Affiliation
|
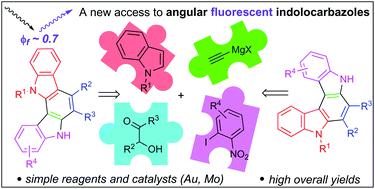
A straightforward and efficient synthesis of the two less explored types of indolocarbazoles has been developed. Two different processes for the carbazole nucleus preparation, a gold-catalysed regioselective cyclization followed by the dioxomolybdenum-catalysed version of Cadogan reductive cyclization, enables the sequential construction of two carbazole cores. The procedure features total regioselectivity and high overall yields. The required starting α-indol-3-ylalkyl propargylic alcohols are easily and efficiently accessed from commercially available reagents. In addition, the photoluminescent properties of two indolo[2,3-c]carbazoles, with fluorescence quantum yields around 0.7, have been studied.
中文翻译:

通过合并Au和Mo催化直接获得高荧光角吲哚并咔唑
已经开发了两种较少探索的吲哚并咔唑的直接有效合成方法。咔唑核制备的两种不同方法,即金催化的区域选择性环化,然后是二氧钼催化的Cadogan还原环化的形式,使得可以依次构建两个咔唑核。该程序具有区域选择性高和总收率高的特点。所需的起始α-吲哚-3-基烷基炔丙基醇可以容易地和有效地从市售试剂中获得。此外,还研究了两种吲哚[2,3- c ]咔唑的荧光性质,荧光量子产率约为0.7。
更新日期:2020-07-14
中文翻译:

通过合并Au和Mo催化直接获得高荧光角吲哚并咔唑
已经开发了两种较少探索的吲哚并咔唑的直接有效合成方法。咔唑核制备的两种不同方法,即金催化的区域选择性环化,然后是二氧钼催化的Cadogan还原环化的形式,使得可以依次构建两个咔唑核。该程序具有区域选择性高和总收率高的特点。所需的起始α-吲哚-3-基烷基炔丙基醇可以容易地和有效地从市售试剂中获得。此外,还研究了两种吲哚[2,3- c ]咔唑的荧光性质,荧光量子产率约为0.7。











































 京公网安备 11010802027423号
京公网安备 11010802027423号