当前位置:
X-MOL 学术
›
J. Mater. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Combining surface chemistry modification and in situ small-angle scattering characterization to understand and optimize the biological behavior of nanomedicines.
Journal of Materials Chemistry B ( IF 6.1 ) Pub Date : 2020-06-10 , DOI: 10.1039/d0tb01167c Marine Le Goas 1 , Tom Roussel 1 , Maria Kalbazova 1 , David Carrière 1 , Elodie Barruet 1 , Valerie Geertsen 1 , Giulia C Fadda 2 , Fabienne Testard 1 , Geraldine Carrot 1 , Jean-Philippe Renault 1
Journal of Materials Chemistry B ( IF 6.1 ) Pub Date : 2020-06-10 , DOI: 10.1039/d0tb01167c Marine Le Goas 1 , Tom Roussel 1 , Maria Kalbazova 1 , David Carrière 1 , Elodie Barruet 1 , Valerie Geertsen 1 , Giulia C Fadda 2 , Fabienne Testard 1 , Geraldine Carrot 1 , Jean-Philippe Renault 1
Affiliation
|
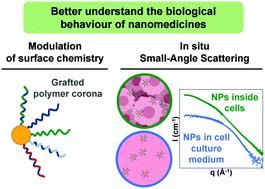
Nanomedicines are considered as promising therapeutics for cancer treatment. However, clinical translation is still scarce, partly because their biological behavior is not well understood. Extracting general guidelines from the great variety of nanoparticles and conditions studied is indeed difficult, and relevant techniques are lacking to obtain in situ information. Here, both issues are solved by combining versatile model nanoparticles with in situ tools based on small-angle scattering techniques (SAS). The strategy was to develop a library of nanoparticles and perform systematic study of their interactions with biological systems. Considering the promising properties of gold nanoparticles as cancer therapeutics, polymethacrylate-grafted gold nanoparticles were chosen as models. Modulation of polymer chemistry was shown to change the surface properties while keeping the same structure for all nanoparticles. This unity allowed reliable comparison to extract general principles, while the synthesis versatility enabled to fine-tune the nanoparticles surface properties, especially through copolymerization, and thus to optimize their biological behavior. Two specific aspects were particularly examined: colloidal stability and cell uptake. Positive charges and hydrophobicity were identified as key parameters influencing toxicity and internalization. In situ SAS gave valuable information about nanoparticles evolution in biologically relevant environments. Good colloidal stability was thereby shown in cell culture media, while intracellular transformation and quantity of nanoparticles were monitored, highlighting the potential of these techniques for nanomedicines studies.
中文翻译:

结合表面化学修饰和原位小角度散射表征,以了解和优化纳米药物的生物学行为。
纳米药物被认为是用于癌症治疗的有前途的疗法。但是,临床翻译仍然很少,部分原因是人们对它们的生物学行为还不甚了解。从所研究的各种纳米颗粒和条件中提取一般指导原则确实是困难的,并且缺乏获得原位信息的相关技术。在这里,这两个问题都是通过将通用模型纳米颗粒与原位结合而解决的基于小角度散射技术(SAS)的工具。该策略是开发一个纳米粒子库,并对它们与生物系统的相互作用进行系统的研究。考虑到金纳米颗粒作为癌症治疗剂的前景广阔,选择了聚甲基丙烯酸酯接枝的金纳米颗粒作为模型。结果表明,调节聚合物化学性质可以改变表面性质,同时对所有纳米粒子保持相同的结构。这种统一允许可靠的比较以提取一般原理,而合成的多功能性可以微调纳米颗粒的表面性能,尤其是通过共聚,从而优化其生物学行为。特别检查了两个具体方面:胶体稳定性和细胞摄取。原位SAS提供了有关生物学相关环境中纳米粒子进化的有价值的信息。因此,在细胞培养基中显示出良好的胶体稳定性,同时监测了细胞内转化和纳米颗粒的数量,突显了这些技术在纳米药物研究中的潜力。
更新日期:2020-08-05
中文翻译:

结合表面化学修饰和原位小角度散射表征,以了解和优化纳米药物的生物学行为。
纳米药物被认为是用于癌症治疗的有前途的疗法。但是,临床翻译仍然很少,部分原因是人们对它们的生物学行为还不甚了解。从所研究的各种纳米颗粒和条件中提取一般指导原则确实是困难的,并且缺乏获得原位信息的相关技术。在这里,这两个问题都是通过将通用模型纳米颗粒与原位结合而解决的基于小角度散射技术(SAS)的工具。该策略是开发一个纳米粒子库,并对它们与生物系统的相互作用进行系统的研究。考虑到金纳米颗粒作为癌症治疗剂的前景广阔,选择了聚甲基丙烯酸酯接枝的金纳米颗粒作为模型。结果表明,调节聚合物化学性质可以改变表面性质,同时对所有纳米粒子保持相同的结构。这种统一允许可靠的比较以提取一般原理,而合成的多功能性可以微调纳米颗粒的表面性能,尤其是通过共聚,从而优化其生物学行为。特别检查了两个具体方面:胶体稳定性和细胞摄取。原位SAS提供了有关生物学相关环境中纳米粒子进化的有价值的信息。因此,在细胞培养基中显示出良好的胶体稳定性,同时监测了细胞内转化和纳米颗粒的数量,突显了这些技术在纳米药物研究中的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号