当前位置:
X-MOL 学术
›
Environ. Sci.: Water Res. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reinvestigation of NDMA formation mechanisms from tertiary amines during chloramination: a DFT study
Environmental Science: Water Research & Technology ( IF 3.5 ) Pub Date : 2020-06-10 , DOI: 10.1039/d0ew00098a Shuo Zhang 1, 2, 3, 4, 5 , Yingying Zhou 1, 2, 3, 4, 5 , Yong Dong Liu 1, 2, 3, 4, 5 , Rugang Zhong 1, 2, 3, 4, 5
Environmental Science: Water Research & Technology ( IF 3.5 ) Pub Date : 2020-06-10 , DOI: 10.1039/d0ew00098a Shuo Zhang 1, 2, 3, 4, 5 , Yingying Zhou 1, 2, 3, 4, 5 , Yong Dong Liu 1, 2, 3, 4, 5 , Rugang Zhong 1, 2, 3, 4, 5
Affiliation
|
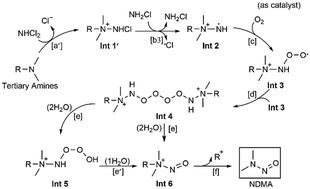
N-Nitrosodimethylamine (NDMA) formation from tertiary amines during chloramination has aroused widespread concern due to its unusually high toxicity and the high NDMA conversion yields of some tertiary amines especially for ranitidine. However, the formation mechanisms have not been fully understood yet. In this study, density functional theory (DFT) calculation as a useful complementary approach to the experiments was used to reinvestigate NDMA formation pathways from tertiary amines during chloramination. The results indicate that the formation of NDMA includes five processes: nucleophilic substitution, aminyl radical generation, oxidation of radical by O2, N-nitroso-compound cation formation and release of NDMA. In the first two processes, the tertiary amines are more likely to first react with dichloramine and the result of which then reacts with monochloramine to generate aminyl radicals. It is elucidated that dichloramine plays a pronounced role in NDMA formation. Moreover, the aminyl radical formation accompanied by the release of chlorine radicals predominates over that by the release of ˙NH2 radicals proposed in the literature mainly due to the lower homolytic bond dissociation energy of N–Cl relative to that of N–H. Importantly, the activation free energy of the release of NDMA from the N-nitroso-compound cation, which is fundamentally related to the previously reported heterolytic ONN(Me)2–R+ bond dissociation energy, was found to be a key indication for distinguishing potential NDMA precursors from tertiary amines. The findings of this work are helpful for expanding the knowledge of NDMA formation mechanisms and predicting potential NDMA precursors during disinfection.
中文翻译:

DFT研究重新调查了叔胺在氯化过程中形成NDMA的机理
叔胺在氯化过程中形成N-亚硝基二甲胺(NDMA)引起了广泛的关注,因为它具有异常高的毒性和某些叔胺的高NDMA转化率,尤其是雷尼替丁。但是,形成机理尚未完全被理解。在这项研究中,密度泛函理论(DFT)计算作为一种有用的实验补充方法,用于重新研究氯化过程中叔胺形成的NDMA形成途径。结果表明,NDMA的形成包括五个过程:亲核取代,氨基自由基生成,自由基被O 2氧化,N-亚硝基化合物阳离子的形成和释放NDMA。在前两个过程中,叔胺更可能先与二氯胺反应,然后其结果与一氯胺反应以生成氨基自由基。阐明了二氯胺在NDMA的形成中起着重要作用。此外,伴随着氯自由基释放的氨基自由基的形成要比文献中提出的释放2NH 2自由基的形成为主,这主要是由于N–Cl的均相键解离能比N–H低。重要的是,N-亚硝基化合物阳离子释放NDMA的活化自由能与先前报道的杂化ONN(Me)2 -R基本相关+键解离能被发现是区分潜在的NDMA前体和叔胺的关键指标。这项工作的发现有助于扩大NDMA形成机理的知识,并预测消毒过程中潜在的NDMA前体。
更新日期:2020-07-31
中文翻译:

DFT研究重新调查了叔胺在氯化过程中形成NDMA的机理
叔胺在氯化过程中形成N-亚硝基二甲胺(NDMA)引起了广泛的关注,因为它具有异常高的毒性和某些叔胺的高NDMA转化率,尤其是雷尼替丁。但是,形成机理尚未完全被理解。在这项研究中,密度泛函理论(DFT)计算作为一种有用的实验补充方法,用于重新研究氯化过程中叔胺形成的NDMA形成途径。结果表明,NDMA的形成包括五个过程:亲核取代,氨基自由基生成,自由基被O 2氧化,N-亚硝基化合物阳离子的形成和释放NDMA。在前两个过程中,叔胺更可能先与二氯胺反应,然后其结果与一氯胺反应以生成氨基自由基。阐明了二氯胺在NDMA的形成中起着重要作用。此外,伴随着氯自由基释放的氨基自由基的形成要比文献中提出的释放2NH 2自由基的形成为主,这主要是由于N–Cl的均相键解离能比N–H低。重要的是,N-亚硝基化合物阳离子释放NDMA的活化自由能与先前报道的杂化ONN(Me)2 -R基本相关+键解离能被发现是区分潜在的NDMA前体和叔胺的关键指标。这项工作的发现有助于扩大NDMA形成机理的知识,并预测消毒过程中潜在的NDMA前体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号