当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Triphenylamine/carbazole-modified ruthenium(ii) Schiff base compounds: synthesis, biological activity and organelle targeting.
Dalton Transactions ( IF 3.5 ) Pub Date : 2020-06-10 , DOI: 10.1039/d0dt01547d Shujiao Chen 1 , Xicheng Liu , Jie Huang , Xingxing Ge , Qinghui Wang , Meimei Yao , Yue Shao , Tong Liu , Xiang-Ai Yuan , Laijin Tian , Zhe Liu
Dalton Transactions ( IF 3.5 ) Pub Date : 2020-06-10 , DOI: 10.1039/d0dt01547d Shujiao Chen 1 , Xicheng Liu , Jie Huang , Xingxing Ge , Qinghui Wang , Meimei Yao , Yue Shao , Tong Liu , Xiang-Ai Yuan , Laijin Tian , Zhe Liu
Affiliation
|
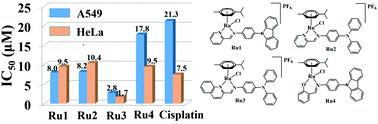
Four triphenylamine/carbazole-modified half-sandwich ruthenium(II) compounds [(η6-p-cymene)Ru(N/O^N)Cl]0/+ with Schiff base chelating ligands (N/O^N) are synthesized and characterized. The introduction of Schiff base units effectively increases the antitumor activity of these compounds (IC50: 1.70 ± 0.56–17.75 ± 3.10 μM), which, meanwhile, can inhibit the metastasis of tumor cells effectively. These compounds follow an energy-dependent cellular uptake mechanism, mainly accumulate in lysosomes to destroy their integrity, and then eventually promote apoptosis. In addition, these compounds can induce an increase of intracellular reactive oxygen species (ROS) levels and provide an antitumor mechanism of oxidation, which is confirmed by the decrease of mitochondrial membrane potential (MMP) and the catalytic oxidation of the coenzyme nicotinamide-adenine dinucleotide (NADH). All these indicate that these ruthenium(II) compounds are expected to be dual-functional antitumor agents: anti-metastasis and lysosomal damage.
中文翻译:

三苯胺/咔唑改性的钌(ii)Schiff碱化合物:合成,生物活性和细胞器靶向。
四个三苯基胺/咔唑改性的半夹心钌(II)的化合物,[(η 6 - p -cymene)的Ru(N / O ^ N)CL] 0 / +与席夫碱的螯合配体(N / O ^ N)进行合成和特点。引入席夫碱单元可有效提高这些化合物的抗肿瘤活性(IC 50:1.70±0.56–17.75±3.10μM),同时可以有效抑制肿瘤细胞的转移。这些化合物遵循能量依赖性细胞摄取机制,主要在溶酶体中积累以破坏其完整性,然后最终促进细胞凋亡。此外,这些化合物可诱导细胞内活性氧(ROS)水平升高,并提供抗氧化的氧化机制,这可通过线粒体膜电位(MMP)的降低和辅酶烟酰胺-腺嘌呤二核苷酸的催化氧化来证实(NADH)。所有这些表明,这些钌(II)化合物有望成为双重功能的抗肿瘤药:抗转移和溶酶体损害。
更新日期:2020-06-29
中文翻译:

三苯胺/咔唑改性的钌(ii)Schiff碱化合物:合成,生物活性和细胞器靶向。
四个三苯基胺/咔唑改性的半夹心钌(II)的化合物,[(η 6 - p -cymene)的Ru(N / O ^ N)CL] 0 / +与席夫碱的螯合配体(N / O ^ N)进行合成和特点。引入席夫碱单元可有效提高这些化合物的抗肿瘤活性(IC 50:1.70±0.56–17.75±3.10μM),同时可以有效抑制肿瘤细胞的转移。这些化合物遵循能量依赖性细胞摄取机制,主要在溶酶体中积累以破坏其完整性,然后最终促进细胞凋亡。此外,这些化合物可诱导细胞内活性氧(ROS)水平升高,并提供抗氧化的氧化机制,这可通过线粒体膜电位(MMP)的降低和辅酶烟酰胺-腺嘌呤二核苷酸的催化氧化来证实(NADH)。所有这些表明,这些钌(II)化合物有望成为双重功能的抗肿瘤药:抗转移和溶酶体损害。











































 京公网安备 11010802027423号
京公网安备 11010802027423号