当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The influence of ceria on Cu/TiO2 catalysts to produce abundant oxygen vacancies and induce highly efficient CO oxidation
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2020-06-10 , DOI: 10.1039/d0cy00792g Ching-Shiun Chen 1, 2, 3, 4, 5 , Tse-Ching Chen 3, 4, 5, 6 , Hung-Chi Wu 1, 2, 3, 4 , Jia-Huang Wu 1, 2, 3, 4 , Jyh-Fu Lee 4, 7, 8
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2020-06-10 , DOI: 10.1039/d0cy00792g Ching-Shiun Chen 1, 2, 3, 4, 5 , Tse-Ching Chen 3, 4, 5, 6 , Hung-Chi Wu 1, 2, 3, 4 , Jia-Huang Wu 1, 2, 3, 4 , Jyh-Fu Lee 4, 7, 8
Affiliation
|
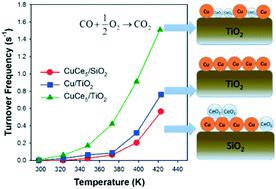
Ce and Cu species deposited on TiO2 (CuCex/TiO2) are employed as catalysts for CO oxidation. The CuCex/TiO2 catalysts can apparently provide a higher turnover frequency (TOF) rate and lower activation energy than a Cu/TiO2 catalyst without a Ce additive. Compared to a CuCe/SiO2 catalyst, TiO2 as a catalyst support can promote a high surface ratio of Cu/Ce and the formation of small CeO2 particles on the Cu/TiO2 catalysts. The presence of a Cu–CeO2 interaction on the CuCex/TiO2 catalysts may increase the likelihood of an increase in the concentration of Ce3+ species and an abundance of oxygen vacancies with the CeO2 particles. The abundance of oxygen vacancies on the CuCex/TiO2 catalysts may result in a high catalytic rate for CO oxidation. Furthermore, the oxygen vacancies can significantly activate the oxygen reactant, thus acting as an oxygen supplier and further catalyzing CO oxidation. The activated oxygen molecule can form active oxygen and/or lattice oxygen to oxidize CO on the Cu surface. On the other hand, a bidentate formate intermediate generated from CO adsorbed on the CuCex/TiO2 surface is also an important factor for high CO oxidation activity. Adsorbed CO at an interface between Cu and CeO2 can combine with nearby active oxygen to form a bidentate formate intermediate that can react with CO to form CO2. In this work, catalyst characterization was performed and reaction kinetics and reaction mechanisms were investigated and correlated to the reaction rate of CO oxidation.
中文翻译:

二氧化铈对Cu / TiO2催化剂产生大量氧空位并诱导高效CO氧化的影响
沉积在TiO 2(CuCe x / TiO 2)上的Ce和Cu物种被用作CO氧化的催化剂。与没有Ce添加剂的Cu / TiO 2催化剂相比,CuCe x / TiO 2催化剂显然可以提供更高的周转频率(TOF)速率和更低的活化能。与CuCe / SiO 2催化剂相比,作为催化剂载体的TiO 2可以促进Cu / Ce的高表面比,并促进在Cu / TiO 2催化剂上形成小的CeO 2颗粒。在CuCe x / TiO 2上存在Cu–CeO 2相互作用催化剂可能会增加Ce 3+物种的浓度增加以及CeO 2颗粒中大量氧空位的可能性。CuCe x / TiO 2催化剂上大量的氧空位可导致高的CO氧化催化速率。此外,氧空位可以显着活化氧反应物,从而充当氧供应者并进一步催化CO氧化。活化的氧分子可以形成活性氧和/或晶格氧以氧化Cu表面上的CO。另一方面,由CO生成的二齿甲酸盐中间体吸附在CuCe x / TiO 2上表面也是高CO氧化活性的重要因素。在Cu和CeO 2之间的界面处吸附的CO可以与附近的活性氧结合形成可与CO反应形成CO 2的双齿甲酸酯中间体。在这项工作中,进行了催化剂表征,研究了反应动力学和反应机理,并将其与CO氧化反应速率相关。
更新日期:2020-07-06
中文翻译:

二氧化铈对Cu / TiO2催化剂产生大量氧空位并诱导高效CO氧化的影响
沉积在TiO 2(CuCe x / TiO 2)上的Ce和Cu物种被用作CO氧化的催化剂。与没有Ce添加剂的Cu / TiO 2催化剂相比,CuCe x / TiO 2催化剂显然可以提供更高的周转频率(TOF)速率和更低的活化能。与CuCe / SiO 2催化剂相比,作为催化剂载体的TiO 2可以促进Cu / Ce的高表面比,并促进在Cu / TiO 2催化剂上形成小的CeO 2颗粒。在CuCe x / TiO 2上存在Cu–CeO 2相互作用催化剂可能会增加Ce 3+物种的浓度增加以及CeO 2颗粒中大量氧空位的可能性。CuCe x / TiO 2催化剂上大量的氧空位可导致高的CO氧化催化速率。此外,氧空位可以显着活化氧反应物,从而充当氧供应者并进一步催化CO氧化。活化的氧分子可以形成活性氧和/或晶格氧以氧化Cu表面上的CO。另一方面,由CO生成的二齿甲酸盐中间体吸附在CuCe x / TiO 2上表面也是高CO氧化活性的重要因素。在Cu和CeO 2之间的界面处吸附的CO可以与附近的活性氧结合形成可与CO反应形成CO 2的双齿甲酸酯中间体。在这项工作中,进行了催化剂表征,研究了反应动力学和反应机理,并将其与CO氧化反应速率相关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号