当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Visible-Light-Driven Triplet Sensitization of Polycyclic Aromatic Hydrocarbons Using Thionated Perinones.
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2020-06-09 , DOI: 10.1021/acs.jpclett.0c01634 Jonathan R Palmer 1 , Kaylee A Wells 1 , James E Yarnell 1, 2 , Joseph M Favale 1 , Felix N Castellano 1
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2020-06-09 , DOI: 10.1021/acs.jpclett.0c01634 Jonathan R Palmer 1 , Kaylee A Wells 1 , James E Yarnell 1, 2 , Joseph M Favale 1 , Felix N Castellano 1
Affiliation
|
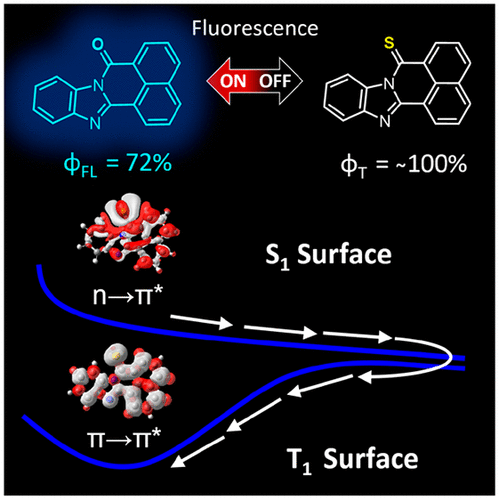
Metal-free chromophores that efficiently generate triplet excited states represent promising alternatives with respect to transition metal-containing photosensitizers, such as those featuring metal-to-ligand charge transfer excited states. However, such molecular constructs have remained underexplored due to the unclear relationship(s) between molecular structure and efficient/rapid intersystem crossing. In this regard, we present a series of three thionated perinone chromophores serving as a newly conceived class of heavy metal-free triplet photosensitizers. We demonstrate that thionation of the lone C═O substituent in each highly fluorescent perinone imparts red-shifted absorbance bands that maintain intense extinction coefficients across the visible spectrum, as well as unusually efficient triplet excited state formation as inferred from the measured singlet O2 quantum yields at 1270 nm (ΦΔ = 0.78–1.0). Electronic structure calculations revealed the emergence of a low energy S1 (n → π*) excited state in the proximity of a slightly higher energy S2 (π → π*) excited state. The distinct character in each of the two lowest-lying singlet state manifolds resulted in the energetic inversion of the corresponding triplet excited states due to differences in electron exchange interactions. Rapid S1 → T1 intersystem crossing was thereby facilitated in this manner through spin–orbit coupling as predicted by the El Sayed rules. The lifetimes of the resultant triplet excited states persisted into the microsecond time regime, as measured by transient absorbance spectroscopy, enabling effective bimolecular triplet sensitization of some common polycyclic aromatic hydrocarbons. The synthetically facile interchange of a single O atom to an S atom in the investigated perinones resulted in marked changes to their photophysical properties, namely, conversion of dominant singlet state fluorescence in the former to long-lived triplet excited states in the latter. The combined results suggest a general strategy for accessing long-lived triplet excited states in organic chromophores featuring a lone C═O moiety residing within its structure, valuable for the design of metal-free triplet photosensitizers.
中文翻译:

可见光驱动的三重态敏化的多环芳烃使用硫代的Perinones。
有效地产生三重态激发态的无金属发色团代表了含过渡金属的光敏剂的有前途的替代品,例如那些具有金属到配体电荷转移激发态的光敏剂。然而,由于分子结构与有效/快速系统间杂交之间的不清楚关系,此类分子构建体仍未得到充分研究。在这方面,我们介绍了一系列的三个亚硫酰紫苏酮生色团,它们是新构思的不含重金属的三重态光敏剂。我们证明了在每个高度荧光的Perinone中,孤独的C═O取代基的硫磺化赋予红移的吸收带,该吸收带在可见光谱范围内保持强烈的消光系数,2个在1270纳米的量子产率(Φ Δ = 0.78-1.0)。电子结构计算表明,在能量稍高的S 2(π→π*)激发态附近出现了低能量S 1(n →π*)激发态。由于电子交换相互作用的不同,两个最低的单重态流形的每一个中的独特特征导致相应的三重态激发态的能量反转。快速S 1 →T 1从而通过El Sayed规则预测的自旋轨道耦合以这种方式促进了系统间交叉。如通过瞬态吸收光谱法所测量的,所得三重态激发态的寿命持续到微秒时间范围,从而使一些常见的多环芳烃能够有效地进行双分子三重态敏化。在所研究的Perinones中,单个O原子与S原子的合成容易互换,导致其光物理性质发生显着变化,即前者的主要单线态荧光转换成后者的长寿命三重态激发态。合并的结果提出了一种在有机发色团中获得长寿命三重态激发态的一般策略,该发色团的结构中存在一个单独的C═O部分,
更新日期:2020-07-02
中文翻译:

可见光驱动的三重态敏化的多环芳烃使用硫代的Perinones。
有效地产生三重态激发态的无金属发色团代表了含过渡金属的光敏剂的有前途的替代品,例如那些具有金属到配体电荷转移激发态的光敏剂。然而,由于分子结构与有效/快速系统间杂交之间的不清楚关系,此类分子构建体仍未得到充分研究。在这方面,我们介绍了一系列的三个亚硫酰紫苏酮生色团,它们是新构思的不含重金属的三重态光敏剂。我们证明了在每个高度荧光的Perinone中,孤独的C═O取代基的硫磺化赋予红移的吸收带,该吸收带在可见光谱范围内保持强烈的消光系数,2个在1270纳米的量子产率(Φ Δ = 0.78-1.0)。电子结构计算表明,在能量稍高的S 2(π→π*)激发态附近出现了低能量S 1(n →π*)激发态。由于电子交换相互作用的不同,两个最低的单重态流形的每一个中的独特特征导致相应的三重态激发态的能量反转。快速S 1 →T 1从而通过El Sayed规则预测的自旋轨道耦合以这种方式促进了系统间交叉。如通过瞬态吸收光谱法所测量的,所得三重态激发态的寿命持续到微秒时间范围,从而使一些常见的多环芳烃能够有效地进行双分子三重态敏化。在所研究的Perinones中,单个O原子与S原子的合成容易互换,导致其光物理性质发生显着变化,即前者的主要单线态荧光转换成后者的长寿命三重态激发态。合并的结果提出了一种在有机发色团中获得长寿命三重态激发态的一般策略,该发色团的结构中存在一个单独的C═O部分,











































 京公网安备 11010802027423号
京公网安备 11010802027423号