当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Water-Triggered Photoinduced Electron Transfer in Acetonitrile-Water Binary Solvent. Solvent Microstructure-Tuned Reactivity of Hydrophobic Solutes.
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-06-10 , DOI: 10.1021/acs.jpcb.0c02635 Anna Lewandowska-Andralojc 1, 2 , Gordon L Hug 3 , Bronislaw Marciniak 1, 2 , Gerald Hörner 4 , Dorota Swiatla-Wojcik 5
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-06-10 , DOI: 10.1021/acs.jpcb.0c02635 Anna Lewandowska-Andralojc 1, 2 , Gordon L Hug 3 , Bronislaw Marciniak 1, 2 , Gerald Hörner 4 , Dorota Swiatla-Wojcik 5
Affiliation
|
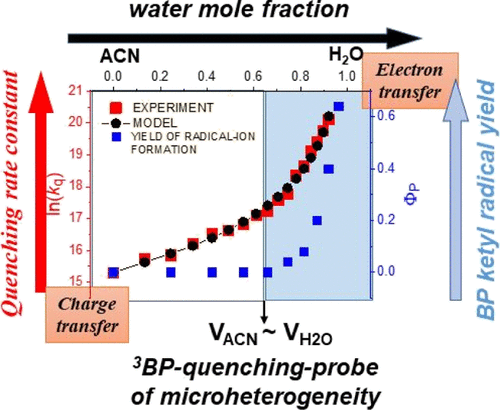
The solvent-composition dependence of quenching triplet states of benzophenone (3BP) by anisole in acetonitrile–water (ACN–H2O) mixtures was investigated by laser flash photolysis over the water mole fraction (xw) increasing from 0 to 0.92. Single exponential decay of 3BP was observed over the whole composition range. The quenching rate constant consistently increased with the water content but increased far more rapidly with xw > 0.7. The water-triggered electron-transfer (ET) mechanism was confirmed by a steeply growing quantum yield of the benzophenone ketyl radical anion, escaping back-ET when the partial water volume exceeded the acetonitrile one. The water-content influence on the 3BP quenching rate was described by a kinetic model accounting for the microheterogeneous structure of the ACN–H2O mixtures and the very different solubility of the reactants in the solvent components. According to the model, the ET mechanism occurs at a rate constant of 1.46 × 109 M–1 s–1 and is presumably assisted by the ACN–H2O hydrogen-bonding interaction.
中文翻译:

乙腈-水二元溶剂中水引发的光诱导电子转移。疏水溶质的溶剂微结构调整反应性。
通过激光闪光光解法研究了水摩尔分数(x w)从0增加到0.92时,苯甲醚在乙腈-水(ACN-H 2 O)混合物中淬灭二苯甲酮(3 BP)的三重态的溶剂组成依赖性。在整个组成范围内观察到3 BP的单指数衰减。淬灭速率常数随水含量持续增加,但随x w的增加更快> 0.7。二苯甲酮酮基自由基阴离子的量子产率陡增证实了水触发的电子转移(ET)机理,当部分水量超过乙腈时,逸出了ET。动力学模型描述了含水量对3 BP淬灭速率的影响,该模型考虑了ACN-H 2 O混合物的微多相结构以及反应物在溶剂组分中的不同溶解度。根据该模型,ET机制以1.46×10 9 M –1 s –1的速率常数发生,并且大概是由ACN–H 2 O氢键相互作用所辅助。
更新日期:2020-07-09
中文翻译:

乙腈-水二元溶剂中水引发的光诱导电子转移。疏水溶质的溶剂微结构调整反应性。
通过激光闪光光解法研究了水摩尔分数(x w)从0增加到0.92时,苯甲醚在乙腈-水(ACN-H 2 O)混合物中淬灭二苯甲酮(3 BP)的三重态的溶剂组成依赖性。在整个组成范围内观察到3 BP的单指数衰减。淬灭速率常数随水含量持续增加,但随x w的增加更快> 0.7。二苯甲酮酮基自由基阴离子的量子产率陡增证实了水触发的电子转移(ET)机理,当部分水量超过乙腈时,逸出了ET。动力学模型描述了含水量对3 BP淬灭速率的影响,该模型考虑了ACN-H 2 O混合物的微多相结构以及反应物在溶剂组分中的不同溶解度。根据该模型,ET机制以1.46×10 9 M –1 s –1的速率常数发生,并且大概是由ACN–H 2 O氢键相互作用所辅助。










































 京公网安备 11010802027423号
京公网安备 11010802027423号