当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effects of Temperature and Solvent Properties on the Liquid–Solid Phase Equilibrium of γ-Pyrazinamide
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-06-10 , DOI: 10.1021/acs.jced.0c00286 Shengzhe Jia 1 , Keke Zhang 1 , Xuxing Wan 1 , Zhenguo Gao 1 , Junbo Gong 1 , Sohrab Rohani 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-06-10 , DOI: 10.1021/acs.jced.0c00286 Shengzhe Jia 1 , Keke Zhang 1 , Xuxing Wan 1 , Zhenguo Gao 1 , Junbo Gong 1 , Sohrab Rohani 2
Affiliation
|
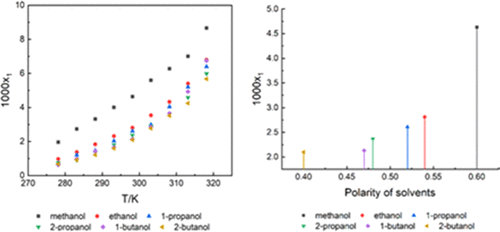
The thermodynamic solubility of γ-pyrazinamide in 13 solvents (methanol, ethanol, 1-propanol, 2-propanol, 1-butanol, 2-butanol, acetone, acetonitrile, water, 1,4-dioxane, methyl acetate, ethyl acetate, and n-propyl acetate) was measured by a dynamic method from 278.15 to 318.15 K. The results were modified by four models (λh equation, Apelblat equation, Wilson model, and nonrandom two-liquid (NRTL) model), and all of the relative standard deviation (RSD) values were less than 6%, indicating that the correlated data is in good agreement with the experiments. The thermodynamic parameters of mixing (ΔmixH, ΔmixG, and ΔmixS) were calculated by the NRTL model. Furthermore, the physicochemical properties of different solvents were investigated to visualize the solvent effects. The results indicated that for the ester and alcohol systems, the “like dissolves like” principle and mass transfer rules were the main factors that affect the solubility, and for other solvents, hydrogen bond donor propensities played the key role.
中文翻译:

温度和溶剂性质对γ-吡嗪酰胺液相固相平衡的影响
γ-吡嗪酰胺在13种溶剂(甲醇,乙醇,1-丙醇,2-丙醇,1-丁醇,2-丁醇,丙酮,乙腈,水,1,4-二恶烷,乙酸甲酯,乙酸乙酯和ñ丙基乙酸酯)用动态法测定从278.15到318.15 K.结果由四个模型(λ进行了修改ħ方程,方程Apelblat,Wilson模型,和非随机双液体(NRTL)模型),并且所有的相对标准偏差(RSD)值小于6%,表明相关数据与实验吻合良好。混合的热力学参数(Δ混合ħ,Δ混合ģ,和Δ混合小号)由NRTL模型计算。此外,研究了不同溶剂的理化性质以可视化溶剂作用。结果表明,对于酯和醇体系,“类比溶解”原理和传质规则是影响溶解度的主要因素,而对于其他溶剂,氢键供体的性质起着关键作用。
更新日期:2020-07-09
中文翻译:

温度和溶剂性质对γ-吡嗪酰胺液相固相平衡的影响
γ-吡嗪酰胺在13种溶剂(甲醇,乙醇,1-丙醇,2-丙醇,1-丁醇,2-丁醇,丙酮,乙腈,水,1,4-二恶烷,乙酸甲酯,乙酸乙酯和ñ丙基乙酸酯)用动态法测定从278.15到318.15 K.结果由四个模型(λ进行了修改ħ方程,方程Apelblat,Wilson模型,和非随机双液体(NRTL)模型),并且所有的相对标准偏差(RSD)值小于6%,表明相关数据与实验吻合良好。混合的热力学参数(Δ混合ħ,Δ混合ģ,和Δ混合小号)由NRTL模型计算。此外,研究了不同溶剂的理化性质以可视化溶剂作用。结果表明,对于酯和醇体系,“类比溶解”原理和传质规则是影响溶解度的主要因素,而对于其他溶剂,氢键供体的性质起着关键作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号