当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Deactivation Mechanism of Cu/SiO2 Catalysts in the Synthesis of Ethylene Glycol via Methyl Glycolate Hydrogenation
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2020-06-10 , DOI: 10.1021/acs.iecr.0c01619 Yujun Zhao 1 , Lingxin Kong 1 , Yuxi Xu 1 , Huijiang Huang 1 , Yaqi Yao 1 , Jingwei Zhang 1 , Shengping Wang 1 , Xinbin Ma 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2020-06-10 , DOI: 10.1021/acs.iecr.0c01619 Yujun Zhao 1 , Lingxin Kong 1 , Yuxi Xu 1 , Huijiang Huang 1 , Yaqi Yao 1 , Jingwei Zhang 1 , Shengping Wang 1 , Xinbin Ma 1
Affiliation
|
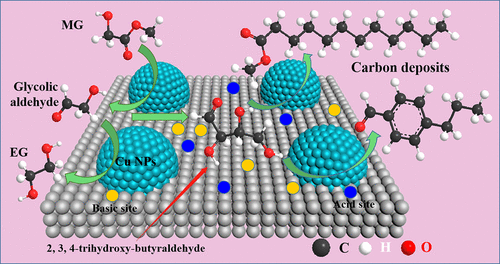
Cu/SiO2 catalysts are prone to deactivation in the dimethyl oxalate (DMO) hydrogenation when high content of methyl glycolate (MG) is produced at a high weight hourly space velocity (WHSV). However, few research studies have focused on the deactivation mechanism, which has become the bottleneck for improving the efficiency of the syngas-to-ethylene glycol (EG) technology. Herein, the deactivation mechanism of copper-based catalysts in the synthesis of EG was studied with MG hydrogenation as the model reaction. The stability test results proved that carrier loss in the form of tetramethoxysilane (TMOS) during the reaction could destroy the structure of the catalysts to some extent. The aggregation of copper nanoparticles (NPs) was also one of the reasons for the deactivation. However, the major factor for the deactivation of the Cu/SiO2 catalyst was deduced to be carbon deposition. The weak acid–base sites of the catalyst led to some side reactions such as alcohol dehydration, condensation, and aromatization via the intermediate of glycolic aldehyde. Larger molecules were formed and accumulated in the pores of the catalyst, leading to the carbon deposition, which caused a rapid deactivation of the catalysts. This deactivation mechanism provides an important guide to develop a highly stable copper-based catalyst for the DMO hydrogenation to EG.
中文翻译:

乙醇酸甲酯加氢合成乙二醇中Cu / SiO 2催化剂的失活机理
铜/二氧化硅2当在高重量时空速度(WHSV)下产生高含量的乙醇酸甲酯(MG)时,草酸二甲酯(DMO)氢化中的催化剂容易失活。但是,很少有研究集中在失活机理上,而失活机理已成为提高合成气制乙二醇(EG)技术效率的瓶颈。在此,以MG氢化为模型反应,研究了EG合成中铜基催化剂的失活机理。稳定性测试结果证明,反应过程中四甲氧基硅烷(TMOS)形式的载流子损失会在一定程度上破坏催化剂的结构。铜纳米颗粒(NPs)的聚集也是失活的原因之一。但是,Cu / SiO失活的主要因素推测2种催化剂为碳沉积。催化剂的弱酸碱位会导致某些副反应,例如乙醇脱水,缩合和通过乙醇醛中间体进行的芳构化。较大的分子形成并聚集在催化剂的孔中,导致碳沉积,这导致催化剂快速失活。这种失活机理为开发高度稳定的铜基催化剂用于DMO加氢至EG提供了重要指导。
更新日期:2020-07-08
中文翻译:

乙醇酸甲酯加氢合成乙二醇中Cu / SiO 2催化剂的失活机理
铜/二氧化硅2当在高重量时空速度(WHSV)下产生高含量的乙醇酸甲酯(MG)时,草酸二甲酯(DMO)氢化中的催化剂容易失活。但是,很少有研究集中在失活机理上,而失活机理已成为提高合成气制乙二醇(EG)技术效率的瓶颈。在此,以MG氢化为模型反应,研究了EG合成中铜基催化剂的失活机理。稳定性测试结果证明,反应过程中四甲氧基硅烷(TMOS)形式的载流子损失会在一定程度上破坏催化剂的结构。铜纳米颗粒(NPs)的聚集也是失活的原因之一。但是,Cu / SiO失活的主要因素推测2种催化剂为碳沉积。催化剂的弱酸碱位会导致某些副反应,例如乙醇脱水,缩合和通过乙醇醛中间体进行的芳构化。较大的分子形成并聚集在催化剂的孔中,导致碳沉积,这导致催化剂快速失活。这种失活机理为开发高度稳定的铜基催化剂用于DMO加氢至EG提供了重要指导。











































 京公网安备 11010802027423号
京公网安备 11010802027423号