当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Comparative Study for Interactions of Sulfate Radical and Hydroxyl Radical with Phenol in the Presence of Nitrite.
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-06-09 , DOI: 10.1021/acs.est.0c02377 Chunyan Chen 1 , Zihao Wu 1 , Shanshan Zheng 2 , Liping Wang 1 , Xizhi Niu 1 , Jingyun Fang 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-06-09 , DOI: 10.1021/acs.est.0c02377 Chunyan Chen 1 , Zihao Wu 1 , Shanshan Zheng 2 , Liping Wang 1 , Xizhi Niu 1 , Jingyun Fang 1
Affiliation
|
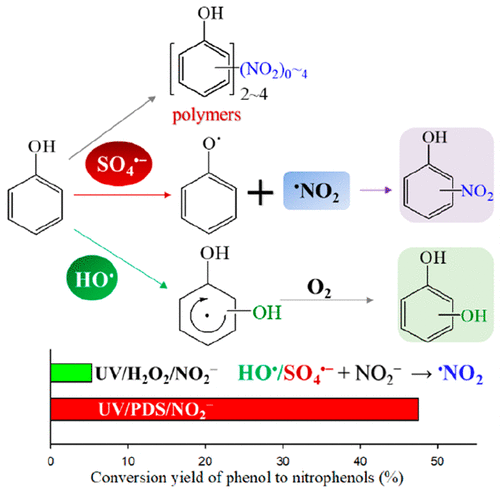
Sulfate radical (SO4•–)- and hydroxyl radical (HO•)-based advanced oxidation processes (AOPs) are effective for the removal of organic pollutants in water treatment. This study compared the interactions of SO4•– and HO• for the transformation of phenol in UV/peroxydisulfate (PDS) and UV/H2O2 with the presence of NO2–, which is widely present in aquatic environments and transforms SO4•– and HO• to •NO2. By using laser flash photolysis, the products of phenol reacting with SO4•– and HO• were demonstrated to be phenoxy radical and phenol-HO-adduct radical, respectively. This result, along with density functional theory (DFT) calculations, indicate that the predominant reaction mechanisms of phenol with SO4•– and HO• with phenol are electron transfer and addition, respectively. The different mechanisms induced the much higher formation of nitrophenols by SO4•– than HO• in the presence of NO2– through the fast combination of phenoxy radicals and •NO2. The conversion yields of phenol to nitrophenols (including 2-nitrophenol and 4-nitrophenol), were 47.5% by SO4•– versus 5.3% by HO• at the experimental conditions. Increasing PDS/H2O2 dosages from 0.2 to 1 mM resulted in a 61.9% increase of nitrophenol conversion yield in UV/PDS/NO2– but a 35.4% decrease of that in UV/H2O2/NO2–. In addition, the significant formation of phenoxy radicals by SO4•– also induced many nitrated polymers in UV/PDS/NO2–, while those induced in UV/H2O2/NO2– were negligible. The significant formation of nitrophenols and nitrated polymers increased the mutagenicity by 860.5% when the removal rate of phenol was 98% by UV/PDS/NO2–. This is the first study to demonstrate the different mechanisms of phenol transformation by SO4•– and HO• in the presence of NO2–.
中文翻译:

亚硝酸盐存在下硫酸根和羟自由基与苯酚相互作用的比较研究。
基于硫酸根(SO 4 •–)和羟基(HO •)的高级氧化工艺(AOPs)可有效去除水处理中的有机污染物。这项研究比较了SO 4 •和HO •在UV /过二硫酸盐(PDS)和UV / H 2 O 2中在存在NO 2 –的情况下酚的转化的相互作用,NO 2 –在水生环境中广泛存在并转化为SO 4 •–和HO •至• NO 2。通过使用激光闪光光解法,苯酚的产物与SO 4 •–和HO •分别被证明是苯氧基和苯酚-HO加合物基团。该结果与密度泛函理论(DFT)的计算结果表明,苯酚与SO 4 •–和HO •与苯酚的主要反应机理分别是电子转移和加成。在NO 2存在下-通过苯氧基和• NO 2的快速结合,不同的机制导致SO 4 •–的硝基酚形成比HO •高得多。SO对苯酚向硝基苯酚(包括2-硝基苯酚和4-硝基苯酚)的转化率为47.5%。在实验条件下为4 •–相对于HO •为5.3%。增加PDS / H 2克ö 2的剂量从0.2至1mM导致UV / PDS / NO硝基苯酚转化率的增加61.9%2 -但在UV / H a的那35.4%的减少2 ö 2 / NO 2 - 。另外,通过使形成显著苯氧自由基的4 • -也诱导许多硝化聚合物在UV / PDS / NO 2 - ,而那些在UV /诱导ħ 2 ö 2 / NO 2 -可以忽略不计。硝基酚和硝化聚合物的显著形成由860.5%提高致突变性时苯酚的除去率为98%的UV / PDS / NO 2 - 。这是第一项研究证明苯酚转化的不同的机制由SO 4 • -和HO •在NO存在2 - 。
更新日期:2020-07-07
中文翻译:

亚硝酸盐存在下硫酸根和羟自由基与苯酚相互作用的比较研究。
基于硫酸根(SO 4 •–)和羟基(HO •)的高级氧化工艺(AOPs)可有效去除水处理中的有机污染物。这项研究比较了SO 4 •和HO •在UV /过二硫酸盐(PDS)和UV / H 2 O 2中在存在NO 2 –的情况下酚的转化的相互作用,NO 2 –在水生环境中广泛存在并转化为SO 4 •–和HO •至• NO 2。通过使用激光闪光光解法,苯酚的产物与SO 4 •–和HO •分别被证明是苯氧基和苯酚-HO加合物基团。该结果与密度泛函理论(DFT)的计算结果表明,苯酚与SO 4 •–和HO •与苯酚的主要反应机理分别是电子转移和加成。在NO 2存在下-通过苯氧基和• NO 2的快速结合,不同的机制导致SO 4 •–的硝基酚形成比HO •高得多。SO对苯酚向硝基苯酚(包括2-硝基苯酚和4-硝基苯酚)的转化率为47.5%。在实验条件下为4 •–相对于HO •为5.3%。增加PDS / H 2克ö 2的剂量从0.2至1mM导致UV / PDS / NO硝基苯酚转化率的增加61.9%2 -但在UV / H a的那35.4%的减少2 ö 2 / NO 2 - 。另外,通过使形成显著苯氧自由基的4 • -也诱导许多硝化聚合物在UV / PDS / NO 2 - ,而那些在UV /诱导ħ 2 ö 2 / NO 2 -可以忽略不计。硝基酚和硝化聚合物的显著形成由860.5%提高致突变性时苯酚的除去率为98%的UV / PDS / NO 2 - 。这是第一项研究证明苯酚转化的不同的机制由SO 4 • -和HO •在NO存在2 - 。











































 京公网安备 11010802027423号
京公网安备 11010802027423号