当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Context-Sensitive Cleavage of Folded DNAs by Loop-Targeting bPNAs.
Biochemistry ( IF 2.9 ) Pub Date : 2020-06-10 , DOI: 10.1021/acs.biochem.0c00362 Yufeng Liang 1 , Shiqin Miao 1 , Jie Mao 1 , Chris DeSantis 1 , Dennis Bong 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-06-10 , DOI: 10.1021/acs.biochem.0c00362 Yufeng Liang 1 , Shiqin Miao 1 , Jie Mao 1 , Chris DeSantis 1 , Dennis Bong 1
Affiliation
|
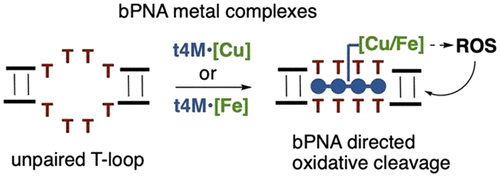
Herein, we demonstrate context-dependent molecular recognition of DNA by synthetic bPNA iron and copper complexes, using oxidative backbone cleavage as a chemical readout for binding. Oligoethylenimine bPNAs displaying iron·EDTA or copper·phenanthroline sites were found to be efficient chemical nucleases for designed and native structured DNAs with T-rich single-stranded domains. Cleavage reactivity depends strongly on structural context, as strikingly demonstrated with DNA substrates of the form (GGGTTA)n. This repeat sequence from the human telomere is known to switch between parallel and antiparallel G-quadruplex (G4) topologies with a change from potassium to sodium buffer: notably, bPNA–copper complexes efficiently cleave long repeat sequences into ∼22-nucleotide portions in sodium, but not potassium, buffer. We hypothesize preferential cleavage of the antiparallel topology (Na+) over the parallel topology (K+) due to the greater accessibility of the TTA loop to bPNA in the antiparallel (Na+) form. Similar ion-sensitive telomere shortening upon treatment with bPNA nucleases can be observed in both isolated and intracellular DNA from PC3 cells by quantitative polymerase chain reaction. Live cell treatment was accompanied by accelerated cellular senescence, as expected for significant telomere shortening. Taken together, the loop-targeting approach of bPNA chemical nucleases complements prior intercalation strategies targeting duplex and quadruplex DNA. Structurally sensitive loop targeting enables discrimination between similar target sequences, thus expanding bPNA targeting beyond simple oligo-T sequences. In addition, bPNA nucleases are cell membrane permeable and therefore may be used to target native intracellular substrates. In addition, these data indicate that bPNA scaffolds can be a platform for new synthetic binders to particular nucleic acid structural motifs.
中文翻译:

通过环靶向bPNAs进行上下文敏感的折叠DNA切割。
在这里,我们证明了使用氧化主链裂解作为结合的化学读数,可以通过合成的bPNA铁和铜配合物对DNA进行上下文相关的分子识别。发现具有铁·EDTA或铜·菲咯啉位点的寡亚乙基亚胺bPNAs是具有富T单链结构域的设计和天然结构DNA的有效化学核酸酶。裂解反应性强烈依赖于结构背景下,作为与以下形式的DNA底物(GGGTTA)惊人地证明Ñ。已知来自人类端粒的重复序列可在平行和反平行G-四链体(G4)拓扑之间切换,从钾缓冲液变为钠缓冲液:值得注意的是,bPNA-铜复合物可有效地将长的重复序列切割成钠中的〜22个核苷酸部分,但不能补钾。我们假设,由于TTA回路在反平行(Na +)中对bPNA的可及性较大,因此反平行(Na +)优先于平行拓扑(K +)裂解。)形式。通过定量聚合酶链反应,可以在PC3细胞的分离DNA和细胞内DNA中观察到用bPNA核酸酶处理后类似的离子敏感性端粒缩短现象。活细胞治疗伴随着加速的细胞衰老,正如预期的端粒显着缩短一样。两者合计,bPNA化学核酸酶的环靶向方法补充了先前针对双链和四链DNA的插入策略。结构敏感的环靶向可区分相似的靶序列,从而将bPNA靶向扩展到简单的寡T序列之外。此外,bPNA核酸酶可透过细胞膜,因此可用于靶向天然细胞内底物。此外,
更新日期:2020-07-07
中文翻译:

通过环靶向bPNAs进行上下文敏感的折叠DNA切割。
在这里,我们证明了使用氧化主链裂解作为结合的化学读数,可以通过合成的bPNA铁和铜配合物对DNA进行上下文相关的分子识别。发现具有铁·EDTA或铜·菲咯啉位点的寡亚乙基亚胺bPNAs是具有富T单链结构域的设计和天然结构DNA的有效化学核酸酶。裂解反应性强烈依赖于结构背景下,作为与以下形式的DNA底物(GGGTTA)惊人地证明Ñ。已知来自人类端粒的重复序列可在平行和反平行G-四链体(G4)拓扑之间切换,从钾缓冲液变为钠缓冲液:值得注意的是,bPNA-铜复合物可有效地将长的重复序列切割成钠中的〜22个核苷酸部分,但不能补钾。我们假设,由于TTA回路在反平行(Na +)中对bPNA的可及性较大,因此反平行(Na +)优先于平行拓扑(K +)裂解。)形式。通过定量聚合酶链反应,可以在PC3细胞的分离DNA和细胞内DNA中观察到用bPNA核酸酶处理后类似的离子敏感性端粒缩短现象。活细胞治疗伴随着加速的细胞衰老,正如预期的端粒显着缩短一样。两者合计,bPNA化学核酸酶的环靶向方法补充了先前针对双链和四链DNA的插入策略。结构敏感的环靶向可区分相似的靶序列,从而将bPNA靶向扩展到简单的寡T序列之外。此外,bPNA核酸酶可透过细胞膜,因此可用于靶向天然细胞内底物。此外,



























 京公网安备 11010802027423号
京公网安备 11010802027423号