当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lifetimes of the Aglycone Substrates of Specifier Proteins, the Autonomous Iron Enzymes That Dictate the Products of the Glucosinolate-Myrosinase Defense System in Brassica Plants.
Biochemistry ( IF 2.9 ) Pub Date : 2020-06-09 , DOI: 10.1021/acs.biochem.0c00358 Leanne E Mocniak , Kyle Elkin 1 , J Martin Bollinger
Biochemistry ( IF 2.9 ) Pub Date : 2020-06-09 , DOI: 10.1021/acs.biochem.0c00358 Leanne E Mocniak , Kyle Elkin 1 , J Martin Bollinger
Affiliation
|
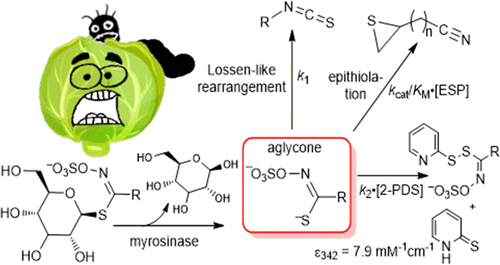
Specifier proteins (SPs) are components of the glucosinolate-myrosinase defense system found in plants of the order Brassicales (brassicas). Glucosinolates (GLSs) comprise at least 150 known S-(β-d-glucopyranosyl)thiohydroximate-O-sulfonate compounds, each with a distinguishing side chain linked to the central carbon. Following tissue injury, the enzyme myrosinase (MYR) promiscuously hydrolyzes the common thioglycosidic linkage of GLSs to produce unstable aglycone intermediates, which can readily undergo a Lossen-like rearrangement to the corresponding organoisothiocyanates. The known SPs share a common protein architecture but redirect the breakdown of aglycones to different stable products: epithionitrile (ESP), nitrile (NSP), or thiocyanate (TFP). The different effects of these products on brassica consumers motivate efforts to understand the defense response in chemical detail. Experimental analysis of SP mechanisms is challenged by the instability of the aglycones and would be facilitated by knowledge of their lifetimes. We developed a spectrophotometric method that we used to monitor the rearrangement reactions of the MYR-generated aglycones from nine GLSs, discovering that their half-lives (t1/2) vary by a factor of more than 50, from <3 to 150 s (22 °C). The t1/2 of the sinigrin-derived allyl aglycone (34 s), which can form the epithionitrile product (1-cyano-2,3-epithiopropane) in the presence of ESP, proved to be sufficient to enable spatial and temporal separation of the MYR and ESP reactions. The results confirm recent proposals that ESP is an autonomous iron-dependent enzyme that intercepts the unstable aglycone rather than a direct effector of MYR. Knowledge of aglycone lifetimes will enable elucidation of how the various SPs reroute aglycones to different products.
中文翻译:

指定蛋白的糖苷配体底物的寿命,该蛋白是决定芸苔属植物中芥子油苷-黑芥子酶防御系统产物的自主铁酶。
指示蛋白(SP)是在十字花科(Brassisicales(brassicas))的植物中发现的芥子油苷-黑芥子酶防御系统的组成部分。芥子油苷(GLS)包含至少150种已知的S-(β- d-吡喃葡萄糖基)硫代氢氧肟酸酯-O-磺酸盐化合物,每个化合物具有与中心碳连接的独特侧链。在组织损伤后,黑芥子酶(MYR)混杂地水解了GLS的常见硫糖苷键,产生了不稳定的糖苷配基中间体,该中间体很容易发生类似类似罗森重排的相应有机异硫氰酸酯。已知的SP都有一个共同的蛋白质结构,但重定向苷元的不同的稳定的产品细分:é pithionitrile(ēSP),Ñ itrile(Ñ SP),或吨hiocyanate(Ť FP)。这些产品对芸苔属消费者的不同影响促使人们努力了解化学反应中的防御反应。SP机制的实验分析受到糖苷配基的不稳定性的挑战,并且通过其寿命的了解将变得更加容易。我们开发了一种分光光度法,用于监测来自9个GLS的MYR生成的糖苷配基的重排反应,发现它们的半衰期(t 1/2)变化范围大于50,从<3到150 s (22℃)。所述吨1/2在ESP存在下可以形成表硫腈产物(1-氰基-2,3-表硫丙烷)的芥子苷烯丙基糖苷(34 s)被证明足以实现MYR和ESP的时空分离反应。该结果证实了最近的提议,即ESP是一种自主的铁依赖性酶,可拦截不稳定的糖苷配基而不是MYR的直接效应子。对糖苷配基寿命的了解将有助于阐明各种SP如何将糖苷配基重新引入不同的产品。
更新日期:2020-07-07
中文翻译:

指定蛋白的糖苷配体底物的寿命,该蛋白是决定芸苔属植物中芥子油苷-黑芥子酶防御系统产物的自主铁酶。
指示蛋白(SP)是在十字花科(Brassisicales(brassicas))的植物中发现的芥子油苷-黑芥子酶防御系统的组成部分。芥子油苷(GLS)包含至少150种已知的S-(β- d-吡喃葡萄糖基)硫代氢氧肟酸酯-O-磺酸盐化合物,每个化合物具有与中心碳连接的独特侧链。在组织损伤后,黑芥子酶(MYR)混杂地水解了GLS的常见硫糖苷键,产生了不稳定的糖苷配基中间体,该中间体很容易发生类似类似罗森重排的相应有机异硫氰酸酯。已知的SP都有一个共同的蛋白质结构,但重定向苷元的不同的稳定的产品细分:é pithionitrile(ēSP),Ñ itrile(Ñ SP),或吨hiocyanate(Ť FP)。这些产品对芸苔属消费者的不同影响促使人们努力了解化学反应中的防御反应。SP机制的实验分析受到糖苷配基的不稳定性的挑战,并且通过其寿命的了解将变得更加容易。我们开发了一种分光光度法,用于监测来自9个GLS的MYR生成的糖苷配基的重排反应,发现它们的半衰期(t 1/2)变化范围大于50,从<3到150 s (22℃)。所述吨1/2在ESP存在下可以形成表硫腈产物(1-氰基-2,3-表硫丙烷)的芥子苷烯丙基糖苷(34 s)被证明足以实现MYR和ESP的时空分离反应。该结果证实了最近的提议,即ESP是一种自主的铁依赖性酶,可拦截不稳定的糖苷配基而不是MYR的直接效应子。对糖苷配基寿命的了解将有助于阐明各种SP如何将糖苷配基重新引入不同的产品。











































 京公网安备 11010802027423号
京公网安备 11010802027423号