Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-06-10 , DOI: 10.1016/j.jmb.2020.06.007 Kei Moritsugu 1 , Yoshihiko Nishino 1 , Akinori Kidera 1
|
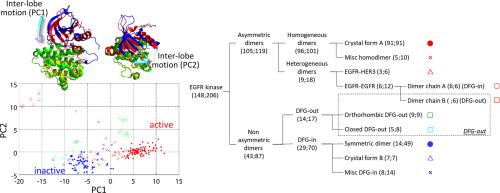
Protein kinases play important roles in cellular signaling and have been one of the best-studied drug targets. The kinase domain of epidermal growth factor receptor (EGFR) is a receptor tyrosine kinase that has been extensively studied for cancer drug discovery and for understanding the unique activation mechanism by dimerization. Here, we analyzed all available 206 crystal structures of the EGFR kinase and the dynamics observed in molecular simulations to identify how these structures are determined. It was found that the arrangement between the N- and C-terminal lobes plays a key role in regulating the kinase structure by sensitively responding to the intermolecular interactions, or the crystal environment. A whole variety of crystal forms in the database is thus reflected in the broad distribution of the inter-lobe arrangement. The configuration of the catalytically important motifs as well as the bound ATP is closely coupled with the inter-lobe motion. When the intermolecular interactions are those of the activating asymmetric dimer, EGFR kinase takes the open-lobe arrangement that constructs the catalytically active configuration.
中文翻译:

瓣间运动以变构方式调节EGFR激酶的结构和功能。
蛋白激酶在细胞信号传导中起重要作用,并且已成为研究最深入的药物靶标之一。表皮生长因子受体(EGFR)的激酶结构域是一种受体酪氨酸激酶,已广泛研究用于癌症药物发现和通过二聚化理解独特的激活机制。在这里,我们分析了EGFR激酶的所有206个可用晶体结构,以及在分子模拟中观察到的动力学,以确定如何确定这些结构。已经发现,通过敏感地响应分子间的相互作用或晶体环境,N末端和C末端叶之间的排列在调节激酶结构中起关键作用。因此,数据库之间的多种晶体形式反映在瓣间排列的广泛分布中。催化重要基序的构型以及结合的ATP与叶间运动密切相关。当分子间的相互作用是活化的不对称二聚体的相互作用时,EGFR激酶采取开叶排列,从而构成催化活性构型。











































 京公网安备 11010802027423号
京公网安备 11010802027423号