Journal of Molecular and Cellular Cardiology ( IF 4.9 ) Pub Date : 2020-06-10 , DOI: 10.1016/j.yjmcc.2020.06.003 Jamie R Bhagwan 1 , Diogo Mosqueira 1 , Karolina Chairez-Cantu 1 , Ingra Mannhardt 2 , Sara E Bodbin 1 , Mine Bakar 1 , James G W Smith 3 , Chris Denning 1
|
Background
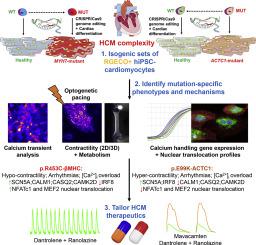
Hypertrophic cardiomyopathy (HCM) is a prevalent and complex cardiovascular condition. Despite being strongly associated with genetic alterations, wide variation of disease penetrance, expressivity and hallmarks of progression complicate treatment. We aimed to characterize different human isogenic cellular models of HCM bearing patient-relevant mutations to clarify genetic causation and disease mechanisms, hence facilitating the development of effective therapeutics.
Methods
We directly compared the p.β-MHC-R453C and p.ACTC1-E99K HCM-associated mutations in human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) and their healthy isogenic counterparts, generated using CRISPR/Cas9 genome editing technology. By harnessing several state-of-the-art HCM phenotyping techniques, these mutations were investigated to identify similarities and differences in disease progression and hypertrophic signaling pathways, towards establishing potential targets for pharmacological treatment. CRISPR/Cas9 knock-in of the genetically-encoded calcium indicator R-GECO1.0 to the AAVS1 locus into these disease models resulted in calcium reporter lines.
Results
Confocal line scan analysis identified calcium transient arrhythmias and intracellular calcium overload in both models. The use of optogenetics and 2D/3D contractility assays revealed opposing phenotypes in the two mutations. Gene expression analysis highlighted upregulation of CALM1, CASQ2 and CAMK2D, and downregulation of IRF8 in p.β-MHC-R453C mutants, whereas the opposite changes were detected in p.ACTC1-E99K mutants. Contrasting profiles of nuclear translocation of NFATc1 and MEF2 between the two HCM models suggest differential hypertrophic signaling pathway activation. Calcium transient abnormalities were rescued with combination of dantrolene and ranolazine, whilst mavacamten reduced the hyper-contractile phenotype of p.ACTC1-E99K hiPSC-CMs.
Conclusions
Our data show that hypercontractility and molecular signaling within HCM are not uniform between different gene mutations, suggesting that a ‘one-size fits all’ treatment underestimates the complexity of the disease. Understanding where the similarities (arrhythmogenesis, bioenergetics) and differences (contractility, molecular profile) lie will allow development of therapeutics that are directed towards common mechanisms or tailored to each disease variant, hence providing effective patient-specific therapy.
中文翻译:

肥厚型心肌病的等基因模型揭示了不同的表型和机制驱动的治疗方法。
背景
肥厚型心肌病 (HCM) 是一种普遍且复杂的心血管疾病。尽管与遗传改变密切相关,但疾病外显率、表现力和进展标志的广泛变化使治疗复杂化。我们旨在表征具有患者相关突变的不同人类 HCM 等基因细胞模型,以阐明遗传因果关系和疾病机制,从而促进有效疗法的开发。
方法
我们直接比较了使用 CRISPR/Cas9 基因组编辑技术生成的人类诱导多能干细胞衍生心肌细胞 (hiPSC-CM) 及其健康同基因对应物中的 p.β-MHC-R453C 和 p.ACTC1-E99K HCM 相关突变。通过利用几种最先进的 HCM 表型技术,对这些突变进行了研究,以确定疾病进展和肥大信号通路的异同,从而建立药物治疗的潜在靶点。CRISPR/Cas9 将基因编码的钙指示剂 R-GECO1.0敲入这些疾病模型中的 AAVS1 基因座,从而产生钙报告基因系。
结果
共聚焦线扫描分析确定了两种模型中的钙短暂性心律失常和细胞内钙超载。光遗传学和 2D/3D 收缩性分析的使用揭示了两种突变中的相反表型。基因表达分析突出了 p.β-MHC-R453C 突变体中CALM1 、CASQ2和CAMK2D的上调和 IRF8的下调,而在 p.ACTC1-E99K 突变体中检测到相反的变化。两种 HCM 模型之间 NFATc1 和 MEF2 核转位的对比表明不同的肥大信号通路激活。丹曲林和雷诺嗪的组合挽救了钙瞬态异常,而马伐康汀降低了 p.ACTC1-E99K hiPSC-CM 的超收缩表型。
结论
我们的数据表明,HCM 内的超收缩性和分子信号在不同基因突变之间并不一致,这表明“一刀切”的治疗低估了疾病的复杂性。了解相似性(心律失常发生、生物能量学)和差异(收缩性、分子谱)所在的位置将允许开发针对共同机制或针对每种疾病变体量身定制的治疗方法,从而提供有效的患者特异性治疗。











































 京公网安备 11010802027423号
京公网安备 11010802027423号