Journal of CO2 Utilization ( IF 7.7 ) Pub Date : 2020-06-10 , DOI: 10.1016/j.jcou.2020.101193 Ossama Al-Juboori , Farooq Sher , Abu Hazafa , Muhammad Kashif Khan , George Z. Chen
|
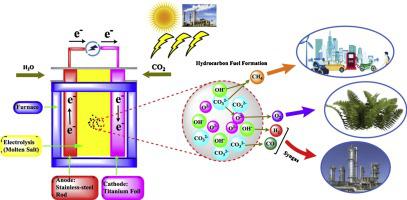
The emission of CO2 has been increasing day by day by growing world population, which resulted in the atmospheric and environmental destruction. Conventionally different strategies, including nuclear power and geothermal energy have been adopted to convert atmospheric CO2 to hydrocarbon fuels. However, these methods are very complicated due to large amount of radioactive waste from the reprocessing plant. The present study investigated the effect of various parameters like temperature (200–500 °C), applied voltage (1.5–3.0 V), and feed gas (CO2/H2O) composition of 1, 9.2, and 15.6 in hydrocarbon fuel formation in molten carbonate (Li2CO3–Na2CO3–K2CO3; 43.5:31.5:25 mol%) and hydroxide (LiOH–NaOH; 27:73 and KOH–NaOH; 50:50 mol%) salts. The GC results reported that CH4 was the predominant hydrocarbon product with a lower CO2/H2O ratio (9.2) at 275 °C under 3 V in molten hydroxide (LiOH–NaOH). The results also showed that by increasing electrolysis temperature from 425 to 500 °C, the number of carbon atoms in hydrocarbon species rose to 7 (C7H16) with a production rate of 1.5 μmol/h cm2 at CO2/H2O ratio of 9.2. Moreover, the electrolysis to produce hydrocarbons in molten carbonates was more feasible at 1.5 V than 2 V due to the prospective carbon formation. While in molten hydroxide, the CH4 production rate (0.80–20.40 μmol/h cm2) increased by increasing the applied voltage from 2.0–3.0 V despite the reduced current efficiencies (2.30 to 0.05%). The maximum current efficiency (99.5%) was achieved for H2 as a by-product in molten hydroxide (LiOH–NaOH; 27:73 mol%) at 275 °C, under 2 V and CO2/H2O ratio of 1. Resultantly, the practice of molten salts could be a promising and encouraging technology for further fundamental investigation for hydrocarbon fuel formation due to its fast-electrolytic conversion rate and no utilization of catalyst.
中文翻译:

可变操作参数对熔融盐电解由CO 2形成烃类燃料的影响
随着世界人口的增长,CO 2的排放量日益增加,这导致了大气和环境的破坏。常规上,已经采用了包括核能和地热能在内的不同策略来将大气中的CO 2转化为碳氢燃料。但是,由于来自后处理厂的大量放射性废物,这些方法非常复杂。本研究调查了碳氢燃料中各种参数(如温度(200–500°C),施加的电压(1.5–3.0 V)和进料气(CO 2 / H 2 O)的组成分别为1、9.2和15.6)的影响熔融碳酸盐(Li 2 CO 3 -Na 2 CO 3–K 2 CO 3;43.5:31.5:25 mol%)和氢氧化物(LiOH-NaOH; 27:73和KOH-NaOH; 50:50 mol%)盐。GC结果表明,CH 4是主要的烃类产物,在275°C和3 V的熔融氢氧化物(LiOH-NaOH)中具有较低的CO 2 / H 2 O比(9.2)。结果还表明,通过将电解温度从425提高到500°C,碳氢化合物中的碳原子数增加到7(C 7 H 16),在CO 2 / H 2下的生产率为1.5μmol/ h cm 2。O比为9.2。此外,由于形成了预期的碳,因此在1.5 V比2 V下电解生成熔融碳酸盐中的碳氢化合物更为可行。在熔融氢氧化物中,尽管电流效率降低(2.30至0.05%),但通过将施加电压从2.0-3.0 V升高,CH 4的生产率(0.80-20.40μmol/ h cm 2)得以提高。在2 V和CO 2 / H 2下,在275°C下于熔融氢氧化物(LiOH-NaOH; 27:73 mol%)中作为副产物H 2达到最大电流效率(99.5%)O比为1。因此,由于其快速的电解转化率且不使用催化剂,因此熔融盐的实践对于烃类燃料的形成进行进一步的基础研究可能是一种有前途的令人鼓舞的技术。



























 京公网安备 11010802027423号
京公网安备 11010802027423号