Journal of Catalysis ( IF 7.3 ) Pub Date : 2020-06-10 , DOI: 10.1016/j.jcat.2020.06.001 Shaoqu Xie , Wanli Zhang , Chuhua Jia , Scott Sergio Go Ong , Cheng Zhang , Shicheng Zhang , Hongfei Lin
|
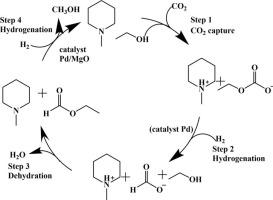
Carbon dioxide (CO2) capture and utilization (CCU) offer a response to greenhouse gas emissions whereas they are often conducted separately, resulting in a high energy demand for the CO2 separation process. The capture reagent chemically binds the CO2 molecule, activating the stubborn CO2 to a more active species. The direct conversion of the captured and activated CO2 to the value-added chemicals will simplify the CO2 utilization process, leading to a strong energy-saving effect by omitting the CO2 separation process. In this paper, the commercial and the self-synthesized noble metal catalysts were evaluated in the hydrogenation of amine captured CO2 in ethanol at 140–165 °C, which is demonstrated to produce the main product methanol in the presence of K3PO4 or using MgO as a catalyst support. The Pd-based catalyst was superior to the Rh, Pt or Ru based catalyst in the hydrogenation of amine captured CO2 towards methanol. The superior Pd/MgO catalyst was characterized by XRD, XPS, SEM, HRTEM, and DRIFTS analysis. NMR measurement and the ATR FTIR measurements were used to determine the initial intermediate to be ethyl carbonate. Mechanistic insight to the methanol formation indicates that the synergistic effect of Pd and a base cascaded the reactions: the reduction of ethyl carbonate to formic acid, the dehydration between formic acid and ethanol to form ethyl formate, and the hydrogenolysis of ethyl formate to methanol and ethanol. The ethyl formate intermediate was hydrogenated to methanol by Pd, wherein basic condition (K3PO4 or MgO) was responsible for the activation of the carbonyl group of the ethyl formate.
中文翻译:

通过整合液相中贵金属的二氧化碳捕获和利用,从源头消除二氧化碳排放
二氧化碳(CO 2)的捕获和利用(CCU)对温室气体的排放做出了响应,而它们通常是分开进行的,因此对CO 2分离过程的能源需求很高。捕获剂化学结合CO 2分子,将顽固的CO 2活化为活性更高的物质。将捕获和活化的CO 2直接转化为增值化学品将简化CO 2的利用过程,并通过省略CO 2分离过程而产生强大的节能效果。在本文中,评估了工业化和自合成的贵金属催化剂在胺捕获的CO加氢中的作用。2在140–165°C的乙醇中溶解,证明在K 3 PO 4存在下或使用MgO作为催化剂载体可生产甲醇的主要产物。在胺捕获的CO 2的氢化中,Pd基催化剂优于Rh,Pt或Ru基催化剂对甲醇。通过XRD,XPS,SEM,HRTEM和DRIFTS分析表征了优良的Pd / MgO催化剂。使用NMR测量和ATR FTIR测量确定初始中间体为碳酸乙酯。对甲醇形成的机理了解表明,Pd和碱的协同作用会级联反应:碳酸乙酯还原为甲酸,甲酸和乙醇之间脱水形成甲酸乙酯,以及甲酸乙酯氢解为甲醇和甲醇。乙醇。甲酸乙酯中间体通过Pd氢化成甲醇,其中碱性条件(K 3 PO 4或MgO)负责甲酸乙酯的羰基活化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号