Journal of Allergy and Clinical Immunology ( IF 11.4 ) Pub Date : 2020-06-10 , DOI: 10.1016/j.jaci.2020.05.038 Mahmoud I Abdel-Aziz 1 , Paul Brinkman 2 , Susanne J H Vijverberg 3 , Anne H Neerincx 2 , Rianne de Vries 4 , Yennece W F Dagelet 2 , John H Riley 5 , Simone Hashimoto 6 , Paolo Montuschi 7 , Kian Fan Chung 8 , Ratko Djukanovic 9 , Louise J Fleming 8 , Clare S Murray 10 , Urs Frey 11 , Andrew Bush 8 , Florian Singer 12 , Gunilla Hedlin 13 , Graham Roberts 9 , Sven-Erik Dahlén 14 , Ian M Adcock 8 , Stephen J Fowler 10 , Karen Knipping 15 , Peter J Sterk 2 , Aletta D Kraneveld 16 , Anke H Maitland-van der Zee 17 , ,
|
Background
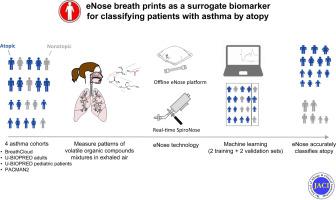
Electronic noses (eNoses) are emerging point-of-care tools that may help in the subphenotyping of chronic respiratory diseases such as asthma.
Objective
We aimed to investigate whether eNoses can classify atopy in pediatric and adult patients with asthma.
Methods
Participants with asthma and/or wheezing from 4 independent cohorts were included; BreathCloud participants (n = 429), Unbiased Biomarkers in Prediction of Respiratory Disease Outcomes adults (n = 96), Unbiased Biomarkers in Prediction of Respiratory Disease Outcomes pediatric participants (n = 100), and Pharmacogenetics of Asthma Medication in Children: Medication with Anti-Inflammatory Effects 2 participants (n = 30). Atopy was defined as a positive skin prick test result (≥3 mm) and/or a positive specific IgE level (≥0.35 kU/L) for common allergens. Exhaled breath profiles were measured by using either an integrated eNose platform or the SpiroNose. Data were divided into 2 training and 2 validation sets according to the technology used. Supervised data analysis involved the use of 3 different machine learning algorithms to classify patients with atopic versus nonatopic asthma with reporting of areas under the receiver operating characteristic curves as a measure of model performance. In addition, an unsupervised approach was performed by using a bayesian network to reveal data-driven relationships between eNose volatile organic compound profiles and asthma characteristics.
Results
Breath profiles of 655 participants (n = 601 adults and school-aged children with asthma and 54 preschool children with wheezing [68.2% of whom were atopic]) were included in this study. Machine learning models utilizing volatile organic compound profiles discriminated between atopic and nonatopic participants with areas under the receiver operating characteristic curves of at least 0.84 and 0.72 in the training and validation sets, respectively. The unsupervised approach revealed that breath profiles classifying atopy are not confounded by other patient characteristics.
Conclusion
eNoses accurately detect atopy in individuals with asthma and wheezing in cohorts with different age groups and could be used in asthma phenotyping.
中文翻译:

eNose呼吸指纹可作为通过特应性对哮喘患者进行分类的替代生物标记。
背景
电子鼻(eNoses)是新兴的即时医疗点工具,可有助于慢性呼吸系统疾病(如哮喘)的亚表型分析。
目的
我们旨在研究eNoses是否可以对小儿和成年哮喘患者的特应性进行分类。
方法
来自4个独立队列的患有哮喘和/或喘息的参与者包括在内;BreathCloud参与者(n = 429),预测呼吸系统疾病结果的无偏见生物标志物(n = 96),预测呼吸系统疾病的无偏见生物标志物(n = 100)和小儿哮喘的药物遗传学:儿童哮喘的药物遗传学:抗抑郁药物-炎症影响2名参与者(n = 30)。特应性被定义为对常见过敏原阳性的皮肤点刺试验结果(≥3mm)和/或阳性的特异性IgE水平(≥0.35kU / L)。通过使用集成的eNose平台或SpiroNose测量呼出的呼吸曲线。根据使用的技术,将数据分为2个训练集和2个验证集。监督数据分析涉及使用3种不同的机器学习算法来对特应性哮喘和非特应性哮喘患者进行分类,并报告接收器工作特征曲线下的面积作为模型性能的度量。此外,通过使用贝叶斯网络执行无监督方法来揭示eNose挥发性有机化合物概况与哮喘特征之间的数据驱动关系。
结果
这项研究纳入了655名参与者(n = 601名成人和学龄期哮喘儿童和54名学龄前儿童喘息[其中68.2%为特应性])的呼吸特征。机器学习模型利用易失性有机化合物的分布来区分特应性和非特应性参与者,其在训练和验证集中的接收器工作特性曲线下的面积分别至少为0.84和0.72。这种无监督的方法显示,对特应性进行分类的呼吸特征不会与其他患者特征混淆。
结论
eNoses可以准确检测哮喘患者和不同年龄组的人群喘息的特应性,可用于哮喘表型分析。











































 京公网安备 11010802027423号
京公网安备 11010802027423号