当前位置:
X-MOL 学术
›
Desalination
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Removal of Cl(−I) from strongly acidic wastewater using NaBiO3: A process of simultaneous oxidation and precipitation
Desalination ( IF 8.3 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.desal.2020.114566 Wenyue Dou , Xingyun Hu , Linghao Kong , Xianjia Peng , Xianliang Wang
Desalination ( IF 8.3 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.desal.2020.114566 Wenyue Dou , Xingyun Hu , Linghao Kong , Xianjia Peng , Xianliang Wang
|
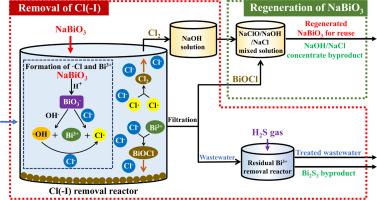
Abstract It was proposed to recycle strongly acidic wastewater generated from nonferrous smelting industries as diluted sulfuric acid after the contaminants being removed, however, Cl(−I) removal from strongly acidic wastewater remains a difficulty. In this work, a process of simultaneous oxidation and precipitation using NaBiO3 for Cl(−I) removal from strongly acidic wastewater was proposed, and 98.1% of Cl(−I) was efficiently removed at the stoichiometric NaBiO3 dosage under 30 °C. The mechanism exploration indicated that Cl(−I) was removed in two ways. In the first way, Cl(−I) was oxidized to generate Cl2: (1) NaBiO3 was dissolved, and the produced BiO3− oxidized Cl(−I) and OH− to Cl and OH, respectively; (2) OH oxidized Cl(−I) to generate Cl−; and (3) Cl combined to produce Cl2. In the second way, Bi3+, the reduced product of BiO3−, reacted with H2O and Cl(−I) to produce BiOCl precipitates. Additionally, through treatment by the NaOH absorption solution for Cl2, almost all of BiOCl product was transformed to the regenerated NaBiO3, which was further employed in Cl(−I) removal. Finally, Cl(−I) was transferred from wastewater to the NaOH/NaCl concentrate. This study offered a theoretical foundation for the development of the method to remove Cl(−I) through oxidation.
中文翻译:

使用 NaBiO3 从强酸性废水中去除 Cl(-I):同时氧化和沉淀的过程
摘要 有色金属冶炼行业产生的强酸性废水提出将污染物去除后作为稀硫酸回收利用,但强酸性废水中Cl(-I)的去除仍存在困难。在这项工作中,提出了一种使用 NaBiO3 同时氧化和沉淀的方法,用于从强酸性废水中去除 Cl(-I),并且在 30 °C 下以化学计量的 NaBiO3 剂量有效去除 98.1% 的 Cl(-I)。机理探索表明 Cl(-I) 以两种方式被去除。在第一种方式中,Cl(-I) 被氧化生成 Cl2: (1) NaBiO3 溶解,生成的 BiO3- 将 Cl(-I) 和 OH- 分别氧化为 Cl 和 OH;(2) OH氧化Cl(-I)生成Cl-;(3) Cl结合生成Cl2。在第二种方式中,Bi3+,BiO3− 的还原产物,与 H2O 和 Cl(-I) 反应生成 BiOCl 沉淀。此外,通过 NaOH 吸收溶液对 Cl2 的处理,几乎所有的 BiOCl 产物都转化为再生的 NaBiO3,进一步用于去除 Cl(-I)。最后,Cl(-I) 从废水中转移到 NaOH/NaCl 浓缩液中。该研究为开发通过氧化去除Cl(-I)的方法提供了理论基础。
更新日期:2020-10-01
中文翻译:

使用 NaBiO3 从强酸性废水中去除 Cl(-I):同时氧化和沉淀的过程
摘要 有色金属冶炼行业产生的强酸性废水提出将污染物去除后作为稀硫酸回收利用,但强酸性废水中Cl(-I)的去除仍存在困难。在这项工作中,提出了一种使用 NaBiO3 同时氧化和沉淀的方法,用于从强酸性废水中去除 Cl(-I),并且在 30 °C 下以化学计量的 NaBiO3 剂量有效去除 98.1% 的 Cl(-I)。机理探索表明 Cl(-I) 以两种方式被去除。在第一种方式中,Cl(-I) 被氧化生成 Cl2: (1) NaBiO3 溶解,生成的 BiO3- 将 Cl(-I) 和 OH- 分别氧化为 Cl 和 OH;(2) OH氧化Cl(-I)生成Cl-;(3) Cl结合生成Cl2。在第二种方式中,Bi3+,BiO3− 的还原产物,与 H2O 和 Cl(-I) 反应生成 BiOCl 沉淀。此外,通过 NaOH 吸收溶液对 Cl2 的处理,几乎所有的 BiOCl 产物都转化为再生的 NaBiO3,进一步用于去除 Cl(-I)。最后,Cl(-I) 从废水中转移到 NaOH/NaCl 浓缩液中。该研究为开发通过氧化去除Cl(-I)的方法提供了理论基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号